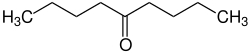
5-nonanone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 5-nonanone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 18 O | |||||||||||||||
| Brief description |
colorless to yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 142.24 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.82 g cm −3 |
|||||||||||||||
| Melting point |
−5 ° C |
|||||||||||||||
| boiling point |
188 ° C |
|||||||||||||||
| Vapor pressure |
0.4 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.419 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
5-Nonanone is a chemical compound from the ketone group .
Extraction and presentation
5-Nonanone can be obtained by reacting tri-n-butylborane with sodium cyanide and then reacting with trifluoroacetic anhydride and hydrogen peroxide. It can also be obtained from 1-butene or valeric acid.
properties
5-Nonanone is a flammable, hardly inflammable, colorless to yellowish liquid that is very sparingly soluble in water.
use
5-Nonanone can be used to make other chemical compounds (such as 5-Nonanketoxime ) and as a solvent.
safety instructions
The vapors of 5-nonanone can form an explosive mixture with air ( flash point 65 ° C, ignition temperature 330 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on 5-nonanone in the GESTIS substance database of the IFA , accessed on April 25, 2017(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 35 ( limited preview in Google Book search).
- ↑ a b Data sheet 5-Nonanone, 98% from Sigma-Aldrich , accessed on April 25, 2017 ( PDF ).
- ↑ Michael B. Smith: Organic Synthesis . Academic Press, 2011, ISBN 978-0-12-415884-9 , pp. 529 ( limited preview in Google Book search).
- ^ W. Carruthers: Some Modern Methods of Organic Synthesis . Cambridge University Press, 1986, ISBN 978-0-521-31117-5 , pp. 308 ( limited preview in Google Book search).
- ^ Paolo Fornasiero, Mauro Graziani: Renewable Resources and Renewable Energy A Global Challenge, Second Edition . CRC Press, 2011, ISBN 978-1-4398-4019-1 , pp. 64 ( limited preview in Google Book search).
- ↑ Entry on dibutyl ketone in the Hazardous Substances Data Bank , accessed on April 25, 2017.