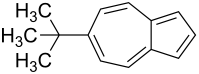
6- tert -Butylazulene
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | 6- tert -Butylazulene | ||||||
| Molecular formula | C 14 H 16 | ||||||
| Brief description |
purple-blue crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 184.28 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
98-99 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
The 6- tert -Butylazulen is a derivative of the azulene .
It was first produced by Thomas Pesel in 1996 from 4- tert- butylpyridine via the Ziegler - Hafner synthesis. The starting material is dissolved in 2,4-dinitrochlorobenzene and treated with dimethylamine . This is followed by reaction with cyclopentadiene and the equivalent amount of sodium .
According to the literature, the chosen reaction path should actually form the 4- tert- butyllazulene, whereby the steric hindrance caused by the tert-butyl group with the five-membered ring of the azulene system should make the formation more difficult. With the analytical methods available at the end of the 1990s, such as two-dimensional NMR spectroscopy , a more than 30 year old error could be cleared up.
Individual evidence
- ↑ a b c Klaus Hafner, Claus Bernhard, Reinhold Müller (1961): On the knowledge of the azulene . Justus Liebig's Annals of Chemistry . 650, 35–41, doi : 10.1002 / jlac.19616500104 (also other chapters).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Thomas Pesel: Representation of azulene carboxylic acid esters and azulenthiocarboxylic acid esters as well as EPR spectroscopic investigation of their radical anions . Dissertation, Univ. Hamburg 1996.