Amphetaminil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
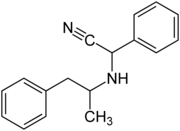
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Amphetaminil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 18 N 2 | |||||||||||||||
| Brief description |
crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 250.3 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
84-87 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Amphetaminil is a mixture of several stereoisomeric compounds and a derivative of amphetamine . Amphetaminil differs from amphetamine only by one substituent on the nitrogen atom or by two substituents on the methyl group on the nitrogen atom compared to methamphetamine .
Pharmacological characterization
Amphetaminil is a psychotropic drug , more precisely a sympathomimetic (see also psychostimulants ). In West Germany, the Berlin company Dr. med. Hans Voigt for medical use under the name AN1. It was used, among other things, to increase drive in seniors.
Misused the drug was as noise and recreationally used.
Metabolism and effects
Amphetaminil is rapidly metabolized to amphetamine in the body , which is why the effects and addiction risks are similar to those of amphetamine.
Stereoisomerism
Amphetaminil contains two stereocenters, so there are four stereoisomers:
- ( R ) -2 - [( R ) -1-Phenylpropan-2-ylamino] -2-phenylacetonitrile (CAS No. 478392-08-4)
- ( S ) -2 - [( S ) -1-Phenylpropan-2-ylamino] -2-phenylacetonitrile (CAS No. 478392-12-0)
- ( R ) -2 - [( S ) -1-Phenylpropan-2-ylamino] -2-phenylacetonitrile (CAS No. 478392-10-8)
- ( S ) -2 - [( R ) -1-Phenylpropan-2-ylamino] -2-phenylacetonitrile (CAS No. 478392-14-2)
Web links
- Information on amphetaminil. catbull.com
Individual evidence
- ^ A b The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals . 14th edition. Merck & Co., Whitehouse Station NJ 2006, ISBN 978-0-911910-00-1 , p. 93.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Too popular with drug addicts . In: Die Zeit , No. 40/1972

