Atomic Mass Evaluation
Since the 1970s, a new, consistent and comprehensive set of the atomic masses of all known nuclides has been made available from time to time under the project name Atomic Mass Evaluation (AME) (German about assessment / evaluation of atomic masses ) . The AME assessments are under the auspices of the C2 Commission of the International Union for Pure and Applied Physics (IUPAP) . The C2 Commission has been responsible for symbols, units, nomenclature, atomic masses and fundamental constants since 1931.
The work is carried out by the Atomic Mass Data Center (AMDC) . Until 2013 it was located in the Center de Spectrométrie Nucléaire et de Spectrométrie de Masse in Orsay near Paris. The AMDC has been continued in the Institute of Modern Physics, Chinese Academy of Sciences in Lanzhou (China) since 2013 . The AMDC collects measured values of the atomic mass and related physical quantities, evaluates their quality, calculates reliable values in a complex mathematical procedure and publishes the estimated atomic mass on a regular basis. These Atomic Mass Evaluations are among the most frequently cited publications in the fields of atomic physics, nuclear physics and nuclear chemistry.
Atomic masses are measured directly in mass spectrometers or are derived from energy measurements on radioactive decays or nuclear reactions . The measured values are carefully evaluated and ultimately processed using the least squares method , taking into account all available experimental data.
Similar to how the CODATA provides the reliability and accessibility of basic physical constants, the Atomic Mass Evaluation does this for atomic masses and related quantities. Like the fundamental physical constants, atomic masses are also natural constants .
The estimated atomic masses play a major role in many areas of the natural sciences. In many branches of physics and chemistry, such as atomic physics , nuclear physics , astrophysics , solid state physics , reactor physics , nuclear technology , physical chemistry , nuclear chemistry, etc., the mass of an atom is the link between the physical quantities of mass density and atomic number density for everyone with matter filled room area. The masses that are given in secondary mass tables, in nuclide maps, etc. worldwide are based on the numerical values of the atomic mass . Interactive calculation aids (tools), e.g. B. those who calculate the Q-values fall back on these atomic masses.
The figure shows the decrease in the relative standard uncertainty of the atomic mass of the nuclide 28 Si in the years between 1933 and 2016. As the trendline shows, the relative standard uncertainty of the atomic mass of this nuclide has decreased by almost one order of magnitude per decade. The atomic mass of 28 Si has a special meaning because the Avogadro project is trying to redefine the SI base unit kilogram of the International System of Units as the mass of a certain number of 28 Si atoms.
About history
The Atomic Mass Data Center continues a tradition started by the chemist and physicist Francis William Aston . The research direction of estimating atomic masses was shaped experimentally and conceptually in particular by the physicist Josef Mattauch and led to its current significance by the experimental nuclear physicist Aaldert Wapstra and his colleague, the physicist Georges Audi.
Presumably for the first time Milton Stanley Livingston and Hans Albrecht Bethe combined mass data from mass spectroscopy, data from induced nuclear reactions and decay data up to the nuclide 40 K. In Germany in the 1930s, atomic masses were systematically collected under the aegis of chemists and an annual report was published in the Reports of the German Chemical Society under the title "The chemical elements and natural atomic types according to the state of isotope and nuclear research" published. The interest in this compilation shifted more and more towards physics and was therefore published in the Physikalische Zeitschrift after 1940 .
The following table, divided into three periods, is based on a historical outline in a work by Georges Audi, which he begins with the sentence: The history of nuclear masses is almost as old as that of nuclear physics itself.
year Authors Title / remark 1933 FW Aston Mass-spectra and isotopes 1935 HA Bethe Masses of Light Atoms from Transmutation Data 1937 MS Livingston, HA Bethe Combined assessment: energies + mass 1940 O. Hahn, S. Flügge, J. Mattauch The chemical elements and natural types of atoms ... 1943 S. Flügge, J. Mattauch The chemical elements and natural types of atoms ... 1946 G. Seaborg The Plutonium project table 1948 AH Wapstra Table of atomic nuclei 1955 AH Wapstra, JR Huizenga Isotopic masses 1956 J. Mattauch et al. The masses of light nuclides 1957 J. Mattauch, F. Everling Masses of atoms of A <40 1960 F. Everling et al. Relative nuclidic masses 1962 LA Koenig et al. 1961 nuclidic mass table 1965 JHE Mattauch et al. 1964 atomic mass table 1971 AH Wapstra, MB Gove The 1971 atomic mass evaluation 1977 AH Wapstra, K. Bos The 1977 atomic mass evaluation 1985 AH Wapstra, G. Audi The 1983 atomic mass evaluation 1993 G. Audi, AH Wapstra The 1993 atomic mass evaluation 2003 AH Wapstra, G. Audi, et al. The AME2003 atomic mass evaluation 2012 G. Audi et al., M. Wang et al. The AME2012 atomic mass evaluation 2016 WJ Huang et al., M. Wang et al. The AME2016 atomic mass evaluation
In the 1950s, mathematical methods were developed using the least squares method to estimate atomic masses that were measured several times with varying degrees of accuracy and / or came from various sources (mass spectroscopy, induced nuclear reactions and decay data). The first modern assessment of atomic masses in this sense was published by AH Wapstra and JR Huizenga in 1955. In 1971, Atomic mass evaluation was also found to be a “popular” title that has been preserved to this day.
An alternative collection of atomic masses was started under the project name Atomic Mass Compilation (AMC) and the results were published in 2014 under the title Atomic mass compilation 2012 . One of the authors of this collection, Bernd Pfeiffer from the GSI Helmholtz Center for Heavy Ion Research , was also co-author of the AME2012. This new atomic mass project, in particular the name AMC2012, which can be confused with AME2012, was criticized and it was stated: We would like to stress that the AMC12 is by no means the continuation or an update of the work initiated by AH Wapstra in the 1950's (We would like to emphasize that the AMC12 is by no means a continuation or an update of the work started by AH Wapstra in the 1950s).
Results of an Atomic Mass Evaluation
Recommendations of the Atomic Mass Data Center were usually published as Atomic mass evaluations (AME) at time intervals of around 10 years. The AME1993, AME2003 and AME2012 was followed by the AME2016 at a shorter interval. The atomic mass evaluations contain the atomic masses of all known nuclides (and their related quantities), including the associated standard uncertainties. Atomic mass refers to the mass of a neutral, unbound atom in the nuclear and electronic ground state.
In the current AME2016, mass data from 3436 nuclides were estimated. For tradition and printing reasons, the data is divided into three data groups. A machine-readable ASCII file (mirrored on two additional servers) can be called up for each data group (see below).
In the second part of the AME2016 The AME2016 atomic mass evaluation (II). Tables, graphs and references , the data are divided into two tables, Tables I and III. Table II Influences on primary nuclei shows the most important data contributions for each of the 1207 primary nuclides and their influence on its mass. These data are of little interest to the user and are therefore not discussed here.
Table I, the main table ( atomic mass table ), contains for each nuclide according to N (number of neutrons), Z (number of protons), A (number of nucleons, A = N + Z ), Elt. (Element symbol), orig. (Origin of the values for secondary nuclides) four quantities and their standard uncertainties. All quantities are given in keV, with the exception of the atomic mass itself, which is given in µu (micro- atomic mass units ).
1. Data group
The 1st data group, in Table I Atomic mass table, contains the following values:
Mass excess (Massenexzess), Binding energy per nucleon (Bindungsenergie pro Nukleon), Beta-decay energy (Betazerfallsenergie), Q(β-) = (m(A,Z) - m(A,Z+1))*c2, Atomic mass, die Atommasse in µu.
The data of this group are summarized in the ASCII file mass16.txt . A separate ASCII file mass16round.txt provides rounded values for this ASCII file. The square of the speed of light in vacuum , the conversion factor between mass and the energy equivalent of mass, is omitted from formulas in the AME publications, following an old tradition. In contrast, in the CODATA publications and in this article, a precise linguistic distinction is made between mass and energy equivalent of mass. There are z. B. the following sizes are listed: neutron mass (in kg), neutron mass energy equivalent (in J), neutron mass energy equivalent in MeV, neutron mass in u.
c2
2. Data group
Table III Nuclear-reaction and separation energies contains 12 sizes, divided into two groups:
S(n) = (-m(A,Z) + m(A-1,Z) + mn)*c2 S(p) = (-m(A,Z) + m(A-1,Z-1) + m(1H))*c2 Q(4β-) = (m(A,Z) - m(A,Z+4))*c2 Q(d,α) = (m(A,Z) - m(A-2,Z-1) - m(2H) - m(4He))*c2 Q(p,α) = (m(A,Z) - m(A-3,Z-1) - m(4He) + m(1H))*c2 Q(n,α) = (m(A,Z) - m(A-3;Z-2) - m(4He) + mn)*c2
The AME2016 data of this group are summarized in the ASCII file rct2-16.txt .
3. Data group
Note that in the printed publication in Table III, a printed page with data from the 2nd data group is followed by a printed page with data from the 3rd data group. This data group contains:
S(2n) = (-m(A,Z) + m(A-2,Z) + 2*mn)*c2 S(2p) = (-m(A,Z) + m(A-2,Z-2) + 2*m(1H))*c2 Q(α) = (m(A,Z) - m(A-4,Z-2) - m(4He))*c2 Q(2β-) = (m(A,Z) - m(A,Z+2))*c2 Q(εp) = (m(A,Z) - m(A-1,Z-2) - m(1H))*c2 Q(β-n) = (m(A,Z) - m(A-1,Z+1) - mn)*c2
The data of this group are summarized in the ASCII file rct1-16.txt .
The symbols on the left of the equations in the three data groups mean:
-
Q(β-), and the Q values of single, double, and quadruple beta-minus decays.Q(2β-)Q(4β-) -
Q(α)is the Q value of another spontaneous nuclear reaction, the alpha decay. -
S(n)andS(2n)are the separation energies (binding energies) of the last neutron or the last two neutrons in the atomic nucleus. These quantities, taken with the opposite sign, are equal to the Q values of the nuclear reactions(γ,n)or(γ,2n). The same applies to the proton or to two protons. - For the induced nuclear reactions
(d,α),(p,α)and(n,α)the Q values are explicitly included in the tables. -
Q(εp)the Q value of a (radioactive) electron capture with subsequent proton emission. -
Q(β-n)symbolizes the Q value of a prompt or delayed neutron emission after a β - decay of a nuclide that was formed after a nuclear fission .
The symbols on the right-hand side of the equations mean: m(A,Z)the mass of the nuclide with Anucleons and Zprotons, the mass of the neutron, the masses of the atoms of light and heavy hydrogen or the 4 He atom.
mnm(1H)), m(2H), m(4He)
The * character instead of a value in the tables, both in the journal articles and in the ASCII files, means: Cannot be calculated from the input data. The # sign instead of a decimal point: value and standard uncertainty were estimated from systematic trends.
The following table contains the atomic masses of the nuclides according to AME2016 (p. 030003-2), which are most precisely known.
nuclide Atomic mass (µu) Uncertainty (µu) Relative 1 n 1 008 664.915 823 0.000 491 4.9 · 10 −10 1 H. 1 007 825,032 241 0.000 094 9.3 · 10 −11 2 H 2,014 101,778 114 0.000 122 6.1 · 10 −11 3 H 3,016,049.281,985 0.000 231 7.7 · 10 −11 3 He 3,016,029,322,645 0.000 220 7.3 · 10 −11 4 He 4 002 603,254 130 0.000 063 1.6 · 10 −11 13 C 13 003 354.835 209 0.000 231 1.8 · 10 −11 14 N. 14 003 074.004 460 0.000 207 1.5 · 10 −11 15 N. 15,000 108,898,939 0.000 645 4.3 · 10 −11 16 O 15,994,914,619 598 0.000 173 1.1 · 10 −11 17 O 16 999 131.756 642 0.000 704 4.1 · 10 −11 18 O 17 999 159.612 840 0.000 758 4.2 · 10 −11 19 F 18,998 403.162 882 0.000 927 4.9 · 10 −11 28 Si 27 976 926,534 991 0.000 524 1.9 · 10 −11 29 Si 28 976 494,665 252 0.000 600 2.1 · 10 −11 31 P. 30 973 761,998 625 0.000 724 2.3 · 10 −11
The standard uncertainties are listed under the heading Uncertainty (µu) and the relative standard uncertainties of the atomic masses are listed under Relative .
Standard uncertainties of the AME values
All values of the AME are based on measurements of atomic mass or are the result of an estimate of a systematic trend. The published values are estimated values. The AME-tested at a standard uncertainty ( English Standard uncertainty specified). Atomic masses and uncertainties are determined in a compensation calculation. Mathematically, this type of uncertainty is treated like a standard deviation. As a rule, several measured values are available for the mass of one and the same nuclide, be it from mass spectroscopic measurements or from nuclear reactions. From a mathematical point of view, the system from which a mass value is to be calculated is over-determined. Using the least squares method, the overdetermined system of equations is now solved with the relevant measured values that have been determined and published worldwide up to a key date.
The table above, based on the AME2016 assessment, also contains the relative standard uncertainties of the atomic masses of the nuclides that have been most accurately measured. The nuclide 16 O has with 1.1 · 10 −11 the smallest relative standard uncertainty of all measured atomic masses. Among the stable nuclides, 138 Ce has the greatest relative standard uncertainty with 7.7 · 10 −8 . The two largest measured relative standard uncertainties were found for the extremely short-lived nuclide 4 Li with 5.7 · 10 −5 and the nuclide 17 Na with 6.3 · 10 −5 .
Let be the atomic mass of a nuclide and the associated standard uncertainty. Then the quotient
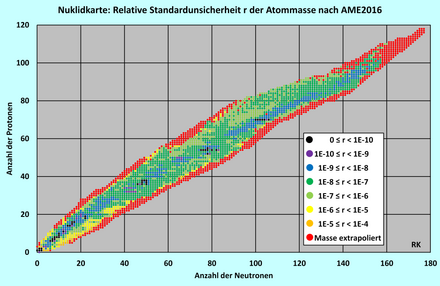
referred to as the relative standard uncertainty . It can easily be calculated from the atomic masses given by the AME2016 and their standard uncertainties for all nuclides, but is not explicitly stated there. The figure shows the relative standard uncertainties of all nuclides, divided into eight classes, in the form of a nuclide map . The nuclides, the atomic mass of which is known with the greatest accuracy, are shown as black dots. Nuclides whose atomic mass and standard uncertainties were not measured but rather extrapolated from systematic trends (the # sign instead of a decimal point) are marked as red dots.
NUBASE
In parallel to the AME, the AMDC started another data collection in 1993, the NUBASE evaluation (NUclear data BASE) and published the first version in 1997. From 2003 it will appear synchronously with the AME. In addition to data for the basic nuclear states of the nuclides, it also contains data for all known isomeric states of the nuclides with half-lives greater than 100 ns. This increases the number of data sets from 3436 (nuclides) in AME2016 to 5625 (nuclides + isomeric states) in NUBASE2016.
NUBASE also uses data for which the AMDC is not directly responsible, the Evaluated Nuclear Structure Data Files (ENSDF). The ENSDF are managed by the National Nuclear Data Center (NNDC) on behalf of the International Network of Nuclear Structure and Decay Data Evaluators (NSDD) , which is sponsored by the International Atomic Energy Agency in Vienna.
The aim of NUBASE is to collect the most important data for each nuclide in a table. These are
- Mass excess, representative of the atomic mass, in keV,
- Excitation energy of the isomeric state in keV,
- Half-life (if unstable), all three quantities each with standard uncertainties,
- Angular momentum and parity J π of the nuclear ground state or isomeric states,
- Isospin T , provided the isomeric state belongs to a multiplet of isobaric analog states (IAS) ,
- Year from which the ENSDF data originate,
- a reference,
- the year of discovery (if known) for the basic and isomeric states and
- Decay modes and branching ratios.
As in the case of AME2016, in addition to the publication itself, a computer-readable ASCII file nubase2016.txt is placed on the web, in which you can quickly find the data you are looking for on a nuclide or its excited states. Note that this ASCII file does not contain any column labels.
Individual evidence
- ↑ C2: Commission on Symbols, Units, Nomenclature, Atomic Masses and Fundamental Constants. Retrieved March 11, 2018 .
- ^ Homepage of the Atomic Mass Data Center. (No longer available online.) Archived from the original on August 13, 2018 ; accessed on August 27, 2018 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Mirror homepage of the Atomic Mass Data Center, the historical Web site of the AMDC. Retrieved March 12, 2018 .
- ^ Mirror homepage of the Atomic Mass Data Center, International Atomic Energy Agency, IAEA. Retrieved March 12, 2018 .
- ^ A b c Francis William Aston: Mass-spectra and isotopes . Arnold, London 1933, p. 170 (English, 248 pp., Babel.hathitrust.org [accessed March 12, 2018]).
- ^ Josef Mattauch: Units of measurement for atomic weights and nuclide masses. In: Journal of Nature Research A . 13, 1958, pp. 572-596 ( online ). - (PDF).
- ^ A b M. Stanley Livingston, Hans Albrecht Bethe: Nuclear Physics C. Nuclear dynamics, experimental . In: Reviews of Modern Physics . tape 9 , no. 3 , 1937, pp. 245 .
- ^ Georges Audi: The history of nuclidic masses and of their evaluation . In: International Journal of Mass Spectrometry . tape 251 , no. 2–3 , 2006, pp. 85–94 , doi : 10.1016 / j.ijms.2006.01.048 ( amdc.in2p3.fr [PDF; accessed on March 13, 2018]).
- ↑ Hans Bethe: Masses of Light Atoms from Transmutation Data . In: Phys. Rev. Band 47 , no. 8 , 1935, pp. 633-634 , doi : 10.1103 / PhysRev.47.633 .
- ↑ Otto Hahn, Siegfried Flügge, Josef Mattauch: The chemical elements and natural types of atoms according to the state of isotope and nuclear research . In: Reports of the German Chemical Society (A and B Series) . tape 73 , no. 1 , 1940.
- ^ Siegfried Flügge, Josef Mattauch: The chemical elements and natural types of atoms according to the status of isotope and nuclear research . In: Physikalische Zeitschrift . tape 44 , 1943, pp. 181 and 391 .
- ^ Glenn T. Seaborg: The Plutonium project table . In: Rev. Mod. Phys. tape 18 , 1946, p. 513 .
- ^ AH Wapstra, Table of atomic nuclei , in L. Rosenfeld, Nuclear Forces , North-Holland, Amsterdam, 1948, p. 497.
- ↑ AH Wapstra, Isotopic masses I. A <34 , Physica 21 (1955) 367th
- ↑ AH Wapstra, Isotopic masses II. 33 <A <202 , Physica 21 (1955) 385.
- ↑ AH Wapstra, JR Huizenga, Isotopic masses III. A> 201 , Physica 21 (1955) 410.
- ↑ J. Mattauch, L. Waldmann, R. Bieri and F. Everling, Ann. Rev. of Nucl. Science 6 (1956) 179.
- ↑ J. Mattauch and F. Everling, Progr. Nucl. Phys. 6 (1957) 233.
- ↑ F. Everling, LA König, JME Mattauch, AH Wapstra: Atomic masses of nuclides for A ≤ 70 . In: Nucl. Phys. A . tape 15 , 1960, pp. 342 .
- ^ F. Everling, LA König, JME Mattauch, AH Wapstra: Relative nuclidic masses . In: Nucl. Phys. A . tape 18 , 1960, p. 529-569 .
- ↑ LA König, JHE Mattauch, AH Wapstra, Nucl. Phys. A 31 (1962) 18.
- ↑ JHE Mattauch, W. Thiele, AH Wapstra, Nucl. Phys. A 67 (1965) 1.
- ↑ AH Wapstra, MB Gove, Nucl. Data Tables 9 (1971) 267.
- ↑ AH Wapstra, K. Bos, Nucl. Data Tables 19 (1977) 177.
- ^ AH Wapstra, G. Audi: The 1983 atomic mass evaluation: (I). Atomic mass table . In: Nuclear Physics A . tape 432 , no. 1 , 1985, pp. 1-54 , doi : 10.1016 / 0375-9474 (85) 90283-0 .
- ^ AH Wapstra, G. Audi: The 1983 atomic mass evaluation: (II). Nuclear reaction and separation energies . In: Nuclear Physics A . tape 432 , no. 1 , 1985, pp. 55-139 , doi : 10.1016 / 0375-9474 (85) 90284-2 .
- ^ K. Bos, G. Audi, AH Wapstra: The 1983 atomic mass evaluation: (III). Systematics of separation and decay energies . In: Nuclear Physics A . tape 432 , no. 1 , 1985, pp. 140-184 , doi : 10.1016 / 0375-9474 (85) 90285-4 .
- ^ AH Wapstra, G. Audi, R. Hoekstra: The 1983 atomic mass evaluation: (IV). Evaluation of input values, adjustment procedures . In: Nuclear Physics A . tape 432 , no. 1 , 1985, pp. 185-362 , doi : 10.1016 / 0375-9474 (85) 90286-6 .
- ↑ G. Audi, AH Wapstra: The 1993 atomic mass evaluation: (I) Atomic mass table . In: Nuclear Physics A . tape 565 , no. 1 , 1993, p. 1–65 , doi : 10.1016 / 0375-9474 (93) 90024-R ( amdc.in2p3.fr [PDF; accessed March 13, 2018]).
- ↑ G. Audi, AH Wapstra: The 1993 atomic mass evaluation: (II) Nuclear-reaction and separation energies . In: Nuclear Physics A . tape 565 , no. 1 , 1993, p. 66-157 , doi : 10.1016 / 0375-9474 (93) 90025-S .
- ^ C. Borcea et al .: The 1993 atomic mass evaluation: (III) Separation and decay energies. Graphs of systematic trends . In: Nuclear Physics A . tape 565 , no. 1 , 1993, p. 158-192 , doi : 10.1016 / 0375-9474 (93) 90026-T .
- ↑ G. Audi, AH Wapstra, M. Dedieu: The 1993 atomic mass evaluation: (IV) Evaluation of input data, adjustment procedures . In: Nuclear Physics A . tape 565 , no. 1 , 1993, p. 193-397 , doi : 10.1016 / 0375-9474 (93) 90027-U .
- ^ G. Audi, AH Wapstra: The 1995 update to the atomic mass evaluation . In: Nuclear Physics A . tape 595 , no. 4 , 1995, p. 409-480 , doi : 10.1016 / 0375-9474 (95) 00445-9 .
- ^ AH Wapstra, G. Audi, C. Thibault: The AME2003 atomic mass evaluation: (I). Evaluation of input data, adjustment procedures . In: Nuclear Physics A . tape 729 , no. 1 , 2003, p. 129–336 , doi : 10.1016 / j.nuclphysa.2003.11.002 ( amdc.in2p3.fr [PDF; accessed on March 13, 2018]).
- ^ G. Audi, AH Wapstra, C. Thibault: The AME2003 atomic mass evaluation: (II). Tables, graphs and references . In: Nuclear Physics A . tape 729 , no. 1 , 2003, p. 337–676 , doi : 10.1016 / j.nuclphysa.2003.11.003 ( amdc.in2p3.fr [PDF; accessed March 13, 2018]).
- ↑ a b G. Audi et al .: The AME2012 atomic mass evaluation (I). Evaluation of input data, adjustment procedures . In: Chinese Physics C . tape 36 , 2012, p. 1287–1602 ( www-nds.iaea.org [PDF; accessed March 11, 2018]).
- ↑ a b M. Wang et al .: The AME2012 atomic mass evaluation (II). Tables, graphs and references . In: Chinese Physics C . tape 36 , 2012, p. 1603–2014 ( www-nds.iaea.org [PDF; accessed March 11, 2018]).
- ↑ a b WJ Huang et al .: The AME2016 atomic mass evaluation (I). Evaluation of input data; and adjustment procedures . In: Chinese Physics C . tape 41 , no. 3 , 2017, p. 30002 ( nds.iaea.org [PDF; accessed March 11, 2018]).
- ↑ a b c d M. Wang et al .: The AME2016 atomic mass evaluation (II). Tables, graphs and references . In: Chinese Physics C . tape 41 , no. 3 , 2017, p. 30003 ( nds.iaea.org [PDF; accessed March 11, 2018]).
- ^ B. Pfeiffer et al .: Atomic mass compilation 2012 . In: Atomic Data and Nuclear Data Tables . tape 100 , no. 2 , 2014, p. 403-535 , doi : 10.1016 / j.adt.2013.06.002 .
- ↑ Georges Audi et al .: Comment on “Atomic mass compilation 2012” by B. Pfeiffer, K. Venkataramaniah, U. Czok, C. Scheidenberger . In: Atomic Data and Nuclear Data Tables . tape 103 , 2015, p. 1–3 ( arxiv.org [PDF; accessed March 13, 2018]).
- ↑ AME2016: ATOMIC MASS ADJUSTMENT, file mass16.txt. (ASCII; 418937 bytes) Retrieved March 11, 2018 .
- ↑ AME2016: ATOMIC MASS ADJUSTMENT, file mass16round.txt. (ASCII; 410681 bytes) Retrieved March 11, 2018 .
- ↑ CODATA2014: Fundamental Physical Constants --- Complete Listing. (ASCII; 38896 bytes) Retrieved March 13, 2018 .
- ↑ AME2016: ATOMIC MASS ADJUSTMENT, File rct2-16.txt. (ASCII; 413404 bytes) Retrieved March 11, 2018 .
- ↑ AME2016: ATOMIC MASS ADJUSTMENT, file rct1-16.txt. (ASCII; 411569 bytes) Retrieved March 11, 2018 .
- ↑ a b G. Audi et al .: The NUBASE 2016 evaluation of nuclear properties . In: Chinese Physics C . tape 41 , no. 3 , 2017, p. 30001 ( nds.iaea.org [PDF; accessed March 11, 2018]).
- ^ G. Audi et al .: The NUBASE evaluation of nuclear and decay properties . In: Nuclear Physics A . tape 729 , no. 1 , 2003, p. 3–128 , doi : 10.1016 / j.nuclphysa.2003.11.001 ( amdc.in2p3.fr [PDF; accessed on March 13, 2018]).
- ↑ Thomas W. Burrows: The evaluated nuclear structure data file: Philosophy, content, and uses . In: Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment . tape 286 , no. 3 , 1990, p. 595-600 , doi : 10.1016 / 0168-9002 (90) 90922-S .
- ↑ ENSDF: Evaluated Nuclear Structure Data File Search and Retrieval. Retrieved March 11, 2018 .
- ↑ AME2016: NUBASE2016, file nubase2016.txt. (ASCII; 677162 bytes) Retrieved March 11, 2018 .