Avogadro's constant
| Physical constant | |
|---|---|
| Surname | Avogadro's constant |
| Formula symbol | |
| value | |
| SI | 6th.022 140 76e23 |
| Uncertainty (rel.) | (exactly) |
| Relation to other constants | |
|
- Universal gas constant - Boltzmann constant - Faraday constant - Elementary charge |
|
| Sources and Notes | |
| Source SI value: CODATA 2018 ( physics.nist.gov ) | |
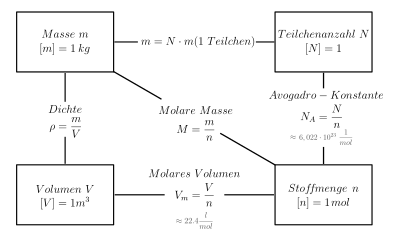
The Avogadro constant indicates how many particles (e.g. atoms of an element or molecules of a chemical compound ) are contained in one mole . It is named after Amedeo Avogadro . The value of Avogadro's constant is
- ,
So a good 602 trillion particles per mole. In general, the following applies
- ,
where is the number of particles and the amount of substance .
The unitless number 6.022 140 76e23 is called theAvogadro number. It was setto this valueas part of therevision of the International System of Units in 2019and has since defined the unit of measurement “mole”. The number was chosen in such a way that 1 mol of particles with a mass ofX atomic mass units(u)each havea total mass ofX grams (g)as precisely as possible.
Until 2019, the mole was defined using the microscopic and macroscopic mass scale: the amount of substance of a total mass X g of particles with particle mass X u was defined as 1 mol. The Avogadro constant was defined as the number of particles in 1 mol and thus a natural constant to be determined experimentally.
Historical and designation
The Avogadro constant has great historical significance for the proof that matter consists of atoms. At the beginning of the 19th century, many scientists viewed atoms as hypothetical particles whose existence was unproven. The certainty of its actual existence was ultimately based in the determination of the Avogadro number using different methods, all of which provided a consistent value.
The Italian physicist Amedeo Avogadro recognized as early as 1811 that equal volumes of different ideal gases contain the same number of molecules at the same pressure and temperature ( Avogadro's law ). With this law he was able to explain measurements that showed that in chemical reactions of gaseous substances the volume ratio of the substances involved can be expressed by simple whole numbers, formulated as Dalton's law of multiple proportions .
In 1865, the Austrian physicist and chemist Josef Loschmidt succeeded for the first time in determining the size of molecules by order of magnitude. Ludwig Boltzmann named the number of molecules in one cubic centimeter of air, derived from Loschmidt's results, as Loschmidt's number. The number of particles per volume under normal conditions is called Loschmidt's constant ( N L or n 0 ). However, the term Loschmidt number is incorrectly used synonymously with Avogadro number , especially in older German-language literature .
It was not until 1909, after the deaths of Loschmidt and Avogadro, that the French chemist Jean-Baptiste Perrin suggested that the number of particles in a mole be referred to as the Avogadro number . The relationship between the Avogadro number in the International System of Units (SI) and the Avogadro constant applies:
Previous definition
Until 2019, the redefinition Avogadro constant was defined as the number of particles in 12 grams of carbon - isotope 12 C in the base state and therefore had a loaded with an uncertainty metric. In addition, the Avogadro constant was dependent on the definition of the base unit “kilogram”.
There are about 60 independent methods for determining the Avogadro constant according to this definition. You can u. a. determine from the surface tension of dilute solutions, such as B. in the oil stain test , from radioactive decay or from the size of the unit cells of a crystal. A precision method for determining the Avogadro constant is the XRCD method ( English X-Ray Crystal Density ). She uses X-ray diffraction experiments on single crystals to determine the size of the unit cell and the number of atoms it contains directly.
The last CODATA value recommended in 2014 before the exact specification was N A = 6.022 140 857 (74)e23 mol −1 . In 2015 the value was experimental at6.022 140 76 (12)e23 mol −1 has been determined. This value was used as the basis for the exact determination.
Applications
The Avogadro constant N A is used to convert between sizes that relate to particle numbers and those that relate to amounts of substance .
-
- Number of particles
- Amount of substance
Relationships with other constants:
literature
- Peter Becker: History and progress in the accurate determination of the Avogadro constant. Rep. Prog. Phys., Vol. 64, 2001, pp. 1945-2008, doi: 10.1088 / 0034-4885 / 64/12/206 .
- Wolfgang Demtröder: Experimental physics 3. Atoms, molecules and solids . Springer, 2009, ISBN 978-3-642-03911-9 , pp. 12–17 ( Chapter 2.2.3: Experimental methods for determining the Avogadro constant in the Google book search [accessed on July 28, 2020]).
Individual evidence
- ↑ CODATA Recommended Values. In: physics.nist.gov. National Institute of Standards and Technology, accessed July 28, 2020 .
- ^ Resolution 1 of the 26th CGPM (2018). In: bipm.org. Bureau International des Poids et Mesures , accessed on July 28, 2020 .
- ↑ The atomic mass unit is defined as 1 ⁄ 12 of the mass of a carbon-12 atom. The mole, in turn, was defined as the amount of 12 g of carbon-12 until 2019.
- ^ Fritz Bosch: History of atomic physics. In: WeltDerPhysik.de. December 7, 2002, accessed July 28, 2020 .
- ^ Joachim Grehn, Joachim Krause: Metzler Physik . Bildungshaus, 2007, ISBN 978-3-507-10710-6 , p. 156 ( limited preview in Google Book Search [accessed July 28, 2020]).
- ↑ Klaus Bethge, Gernot Gruber, Thomas Stöhlker: Physics of Atoms and Molecules. An introduction . John Wiley & Sons, 2012, ISBN 978-3-527-66255-5 , pp. 44–45 ( Chapter 3.2: The Mass in Google Book Search [accessed on July 28, 2020]).
- ↑ Atoms for the kilogram. PTB News 1.2015. In: ptb.de. Physikalisch-Technische Bundesanstalt, April 7, 2015, accessed on July 28, 2020 .
- ↑ Y. Azuma et al. a .: Improved measurement results for the Avogadro constant using a 28 Si-enriched crystal. In: Metrologia , 52, 2015, pp. 360-375, doi: 10.1088 / 0026-1394 / 52/2/360 .