Pentabromodiphenyl ether
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
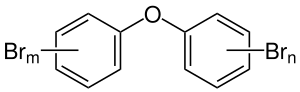
|
||||||||||||||||
| m + n = 5 (five bromine substituents) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Pentabromodiphenyl ether | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 5 Br 5 O | |||||||||||||||
| Brief description |
colorless liquid, also amber colored as a technical product |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 564.69 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.25-2.28 g · cm -3 |
|||||||||||||||
| Melting point |
−7–3 ° C |
|||||||||||||||
| boiling point |
> 200 ° C (decomposition) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Pentabromodiphenyl ether (PentaBDE) is a flame retardant and belongs to the group of polybrominated diphenyl ethers (PBDE).
use
PentaBDE has been used in plastics in the construction industry, in upholstered furniture, in electrical and electronic equipment and in vehicles. The main area of application was polyurethane foams. The annual consumption worldwide in 2001 was estimated at 7500 tons, of which only around 150 tons were used in European industry. A mixture of substances consisting of various congeners was used , including diphenyl ethers with five as well as those with four (tetrabromodiphenyl ether) and six bromine atoms (hexabromodiphenyl ether). The two main components are 2,2 ', 4,4', 5-pentabromodiphenyl ether (BDE-99) and 2,2 ', 4,4'-tetrabromodiphenyl ether (BDE-47).
Only congeners with more than 1% are listed.
Environmental relevance
Due to its toxicity , persistence and tendency to bioaccumulate , the substance was banned in 2004 in the EU , Switzerland, Norway and other countries. In the United States, 10 states have banned the use of PentaBDE by law (California, Hawaii, Illinois, Maine, Maryland, Michigan, New York, Oregon, Rhode Island, and Washington). In Germany, the industry decided in a voluntary agreement in 1986 to forego its use. The tetra- and pentabrominated congeners of commercially used PentaBDE were added to the list of the Stockholm Convention on Persistent Organic Pollutants (POP Convention) in 2009 . Exceptions were granted for some products that contain recycled material. PentaBDE enters the environment through various processes and occurs in environmental compartments such as air, water, soil and river sediments. It is also found in sewage sludge and house dust . House dust levels are much higher in the USA and Canada than in Europe. In a study carried out by the WWF , PentaBDE was found in the blood of MEPs. It can also be found in breast milk. In electrical average concentrations were in 2003 and 2011 carried out studies of 34 ppm or ppm found 2.4, confirming the earlier use of pentaBDE in electrical equipment. The highest concentrations were found in printed circuit boards, as these were flame-retardant with this substance in Asia.
Web links
- Results of the WWF blood test (English; PDF file; 533 kB)
- Toxicological Profile for Polybrominated Diphenyl Ethers (PBDEs )
Individual evidence
- ↑ a b c d e f Entry on pentabromodiphenyl ether in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Entry on Diphenyl ether, pentabromo derivative in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Bromine Science and Environmental Forum: Major Brominated Flame Retardants Volume Estimates - Total Market Demand By region in 2001 ( Memento of 2 April 2015, Internet Archive ) .
- ↑ MJ La Guardia, RC Hale, E. Harvey: Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-retardant Mixtures. In: Environ. Sci. Technol. 40, 2006, pp. 6247-6254, doi: 10.1021 / es060630m .
- ^ RE Alcock, J. Busby: Risk migration and scientific advance: The case of flame-retardant compounds. In: Risk Analysis. 26 (2), 2006, pp. 369-381. PMID 16573627
- ↑ Press Release - COP4 - Geneva, 8 May 2009: Governments unite to step-up reduction on global DDT reliance and add nine new chemicals under international treaty , 2009.
- ↑ B. Kuch, W. Körner, H. Hagenmaier: Monitoring of brominated flame retardants in rivers, sewage and sewage sludge in Baden-Württemberg ( Memento from June 22, 2006 in the Internet Archive ). In: Environment and Health. University of Tübingen, 2001.
- ^ W. Moche, G. Thanner, K. Stephan: Brominated flame retardants in the aquatic environment . (PDF; 523 kB). Federal Environment Agency, Vienna 2004.
- ↑ M. Uhl, P. Hohenblum, S. Scharf, C. Trimbacher: House dust - an indicator for indoor pollution . (PDF; 2.7 MB). Federal Environment Agency, Vienna 2004.
- ↑ WWF Detox Campaign: Bad Blood? A Survey of Chemicals in the Blood of European Ministers . 2004.
- ↑ B. Vieth, T. Rüdiger, B. Ostermann, H. Mielke: Residues of flame retardants in human milk from Germany with special consideration of polybrominated diphenyl ethers . (PDF; 266 kB). Action program “Environment and Health”. Federal Institute for Risk Assessment, Berlin 2005.
- ^ Leo S. Morf, Josef Tremp, Rolf Gloor, Yvonne Huber, Markus Stengele, Markus Zennegg: Brominated Flame Retardants in Waste Electrical and Electronic Equipment: Substance Flows in a Recycling Plant. In: Environmental Science & Technology . 39 (22), 2005, pp. 8691-8699, doi: 10.1021 / es051170k .
- ^ Ruedi Taverna, Rolf Gloor, Urs Maier, Markus Zennegg, Renato Figi, Edy Birchler: Material flows in Swiss electronic waste . Metals, non-metals, flame retardants and polychlorinated biphenyls in small electrical and electronic devices . Federal Office for the Environment , Bern 2017. Environmental status No. 1717: 164 p.







