Bafetinib
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
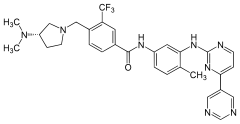
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Bafetinib | |||||||||||||||
| Molecular formula | C 30 H 31 F 3 N 8 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 576.62 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Bafetinib , previously known as INNO-406 , NS-187 and CNS-9 , is an experimental drug from the group of benzamides that is said to be used as a tyrosine kinase inhibitor . It was originally developed by the Japanese company Nippon Shinyaku and out-licensed to Innovive Pharmaceuticals in 2006. Innovive was acquired by CytRx Corp. in June 2008. accepted.
pharmacology
Bafetinib is an inhibitor of tyrosine kinases . It influences the formation of the fusion protein Bcr-Abl as well as that of the enzyme Lyn kinase and is said to be ten times more effective in mice than the established tyrosine kinase inhibitor imatinib .
Clinical development
Bafetinib is currently not approved as a drug for any indication .
The drug is to be developed for the treatment of chronic lymphocytic leukemia (CLL). Bafetinib is in development phase II for this indication (as of June 2011).
Bafetinib is also in phase II for the treatment of hormone-refractory prostate cancer .
The US regulatory authority FDA had Bafetinib end of 2006 the status of a drug orphan (orphan drug) awarded. This status could enable accelerated development and approval.
literature
- E. Weisberg et al. a .: Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. In: Nature Reviews Cancer , 7/2007, pp. 345-356. PMID 17457302
- A. Yokota et al. a .: INNO-406, a novel BCR-ABL / Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph + leukemia cells in the central nervous system and cyclosporine A augments its in vivo activity. (PDF; 735 kB) In: Blood , 109/2007, pp. 306-314. PMID 16954504
Web links
- Entries in the NIH study registry
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ A. Quintás-Cardama et al. a .: Flying under the radar: the new wave of BCR-ABL inhibitors. In: Nature Reviews Drug Discovery 6/2007, pp. 834-848, PMID 17853901 .
- ↑ Press release from January 5, 2006 ( Memento of the original from June 18, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Nippon Shinyaku.Retrieved February 25, 2011.
- ↑ Cytrx Corporation Signs Definitive Agreement to Acquire Innovive Pharmaceuticals, Inc. ( Memento of the original from March 1, 2012 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. drugs.com; Retrieved June 17, 2011.
- ↑ H. Naito et al. a .: In vivo antiproliferative effect of NS-187, a dual Bcr-Abl / Lyn tyrosine kinase inhibitor, on leukemic cells harboring Abl kinase domain mutations. In: Leukemia Research , 30/2006, pp. 1443-1446, PMID 16546254 .
- ↑ Study of Bafetinib as Treatment for Relapsed or Refractory B-Cell Chronic Lymphocytic Leukemia (B-CLL). clinicaltrials.gov; Retrieved June 17, 2011.
- ↑ Study of Bafetinib (INNO-406) as Treatment for Patients With Hormone-Refractory Prostate Cancer (PROACT). clinicaltrials.gov; Retrieved June 17, 2011.
- ↑ Database excerpt from December 27, 2006. ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. Food and Drug Administration; Retrieved September 16, 2009.