Beret
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
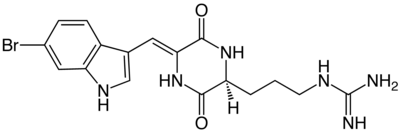
|
|||||||||||||
| General | |||||||||||||
| Surname | Beret | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 17 H 19 BrN 6 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 419.28 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Berettin is a natural brominated alkaloid . Chemically Barrettin containing a diketopiperazine -Grundgerüst selected from the amino acid arginine and a brominated tryptophan - derivative having an additional double bond , is composed.
Occurrence and discovery
Berettin was first isolated in 1986 by Lidgren and Bohlin from the sea sponge Geodia barretti Bowerbank 1858 . They gave the compound the name derived from the sponge, but the structure they postulated was refuted by synthesis in 1987 by Lieberknecht and Griesser. The actual structure of berettin was not revealed until 2002 by Sölter et al. enlightened.
function
Bromine-substituted tryptophan derivatives are not uncommon in marine sponges and other marine invertebrates . At Geodia barretti , these bromine-substituted amino acids fulfill the function of protecting the habitat, for example against barnacles . In the vicinity of the sponges, concentrations of berettin in sea water were measured, which prevent barnacles from settling.
Effect in the human body
The mode of action in the human body is still largely unclear, but Barettin and his homologues show a high therapeutic potential, for example as antimycotics , antimicrobial substances , anthelmintics (worming agents ), insecticides or as chemotherapeutic agents against cancer cells .
Even in low concentrations of 0.25 to 25 µmol, Berettin prevents the settlement of barnacles larvae. Synthetic berettin derivatives show a corresponding effect in even lower concentrations (34 nmol).
At the human serotonin - receptors ( 5-HT receptors ) 5-HT2A, 5-HT2C and 5-HT4 Barettin binds selectively and with similar concentrations as the endogenous serotonin.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ S. Sölter: Identification and synthesis of natural substances from boreal sponges. (PDF; 2.1 MB), dissertation, University of Hamburg, 2004.
- ↑ G. Lidgren, L. Bohlin: Tetrahedron Lett. 1986, 28, 3283-4.
- ^ A. Lieberknecht, H. Griesser: What is the structure of barettin? Novel synthesis of unsaturated diketopiperazines. In: Tetrahedron Letters , 28/1987, pp. 4275-8.
- ↑ S. Sölter: Barettin, revisited? In: Tetrahedron Letters , 43/2002, pp. 3385-6.
- ↑ M. Sjogren et al .: Antifouling activity of brominated cyclopeptides from the marine sponge Geodia barretti. In: J Nat Prod , 67/2004, pp. 368-72, PMID 15043412 .
- ↑ S. Bittner et al: The five bromotryptophans. In: Amino Acids , 33/2007, pp. 19-42, PMID 17031473 .
- ↑ a b E. Hedner et al: Brominated cyclodipeptides from the marine sponge Geodia barretti as selective 5-HT ligands. In: J Nat Prod , 69/2006, pp. 1421-1424, PMID 17067154 .
- ↑ M. Sjögren et al .: Antifouling activity of synthesized peptide analogs of the sponge metabolite barettin. In: Peptides , 27/2006, pp. 2058-2064, PMID 16781016 .
literature
- AL Johnson et al: Synthesis of barettin. In: Tetrahedron , 60/204, pp. 961-5.
- M. Sjogren: Bioactive Compounds from the Marine Sponge Geodia barretti: Characterization, Antifouling Activity and Molecular Targets. , Dissertation, Uppsala University, 2006.
- E. Hedner: Bioactive Compounds in the Chemical Defense of Marine Sponges: Structure-Activity Relationships and Pharmacological Targets. , Dissertation, Uppsala University, 2007.
