Barium propionate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
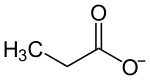 |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Barium propionate | |||||||||||||||
| other names |
Barium propanoate |
|||||||||||||||
| Molecular formula | C 6 H 10 BaO 4 | |||||||||||||||
| Brief description |
colorless voluminous prisms |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 283.47 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| boiling point |
300 ° C (decomposition) |
|||||||||||||||
| pK s value |
4.88 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Barium propionate is the barium salt of propionic acid .
Manufacturing
Barium propionate can be prepared by reaction of barium hydroxide are shown with propionic acid.
properties
Barium propionate forms different hydrates: a monohydrate, which gives off its water of crystallization at 110 ° C, a tri- and a hexahydrate.
When heated dry to 460 ° C, barium propionate decomposes into 3-pentanone and barium carbonate .
Web links
- Entry on barium propionate (IR spectrum). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD
Individual evidence
- ↑ a b c d e A. Renard: "Sur les propionates métalliques" in Comptes rend. hebd. 1887 , 104 , pp. 913-917. Full text
- ↑ WLF Armarego, Christina Li Lin Chai: "Purification of laboratory chemicals," Butterworth-Heinemann, 2003, ISBN 9780750675710 , p 399. ( limited preview in Google Book Search)
- ^ HJ Wing, TJ Thompson: "The solubility of Barium propionate" in J. Am. Chem. Soc 1926 , 48 (1), pp. 104-106, doi : 10.1021 / ja01412a014 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of barium dipropionate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on December 28, 2019, is reproduced from a self-classification by the distributor .
- ↑ U. Hasenkox, S. Hoffmann, R. Waser: "Influence of Precursor Chemistry on the Formation of MTiO3 (M = Ba, Sr) Ceramic Thin Films" in Journal of Sol-Gel Science and Technology 1998 , 12 (2), Pp. 67-79. doi : 10.1023 / A: 1026480027046
- ^ AT Johns: "The Mechanism of Propionic Acid Formation by Veillonella gazogenes" in J. gen. Microbiol. 1951 , 5 , pp. 326-336. doi : 10.1099 / 00221287-5-2-326 , PMID 14832421 .

