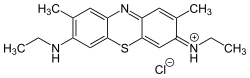
Cationic dyes
Cationic dyes are dyes with a positive charge in the molecule, which preferably produce brilliant colorations with polyacrylonitrile fibers (PAN) or anionic modified polyester fibers . The dyes form a salt bond with the fiber .
Chemical properties
The dyes were formerly known as basic dyes called and they are based on the Color Index as CI Basic Dyes named. However, this classification should no longer be used, since the positively charged structures are not Brønsted acids .
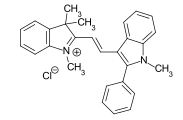
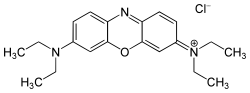
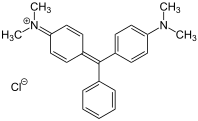
The charge of the cationic dyes can be both delocalized and localized. The molecules with a delocalized charge are methine dyes with a vinylogous amidinium salt structure :
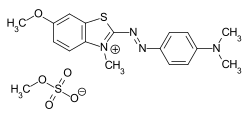
The two terminal nitrogen atoms can also be part of a heterocycle . The double bonds can contain further hetero atoms or also be part of an aromatic system. Any chromophore can be used for the dyes with localized charge - the charge is separated from the chromophoric system by a non-conjugated group. An example is the azo dye C.I. Basic Red 18 .
Examples
Web links
- Rainer Casaretto: Safe and economical dyeing of high-quality textiles made from cationic dyeable fibers ( Memento of March 14, 2006 in the Internet Archive )
- H. Kellett: The Dyeing of Acrylic Fibers . In: Journal of the Society of Dyers and Colourists . tape 84 , no. 5 , May 1968, p. 257–261 , doi : 10.1111 / j.1478-4408.1968.tb02822.x ( PDF ).
Individual evidence
- ^ A b Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 , p. 44 ff . ( limited preview in Google Book search).
- ↑ Entry on cationic dyes. In: Römpp Online . Georg Thieme Verlag, accessed on January 31, 2019.
- ^ A b Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 102 ( limited preview in Google Book search).