Béchamp reduction
The Béchamp reduction is the oldest reaction in industrial organic chemistry for the production of primary aromatic amines from nitroaromatics by reduction with iron and mineral acids. Today, the Béchamp reduction has been largely replaced by catalytic hydrogenation, but is still used in particular in the dye industry and for the production of iron oxide pigments.
Overview reaction
In the simplest case, nitrobenzene is reduced to aniline by the action of the reducing agent hydrochloric acid / iron:
General
The reduction of aromatic nitro compounds to the corresponding amines with iron or iron (II) salts in aqueous hydrochloric acid was discovered in 1854 by Antoine J. Béchamp as a special case of reduction with base metals in acids . In addition to iron, zinc and tin can also be used as reducing agents. In addition, some by-products are produced in this reaction, such as hydroxylamine , hydrazine , hydrazobenzene and azobenzene with which azo dyes can be produced. The possible reaction mechanism was also derived from these components. The usual reaction temperatures are between 80 ° C. and 89 ° C., yields of up to 57% being achieved. It was also found that higher yields are achieved in neutral organic solvents such as acetonitrile or propylene carbonate .
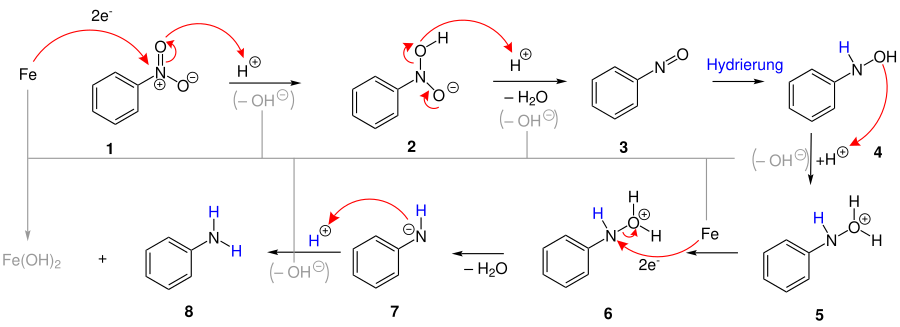
Proposed reaction mechanism
Initially, an acid-catalyzed electron transfer from iron to nitrogen takes place on the nitroaromatic 1 (in the example nitrobenzene ). By absorbing another proton, water splits off, which leads to a shift of electrons from oxygen to positively charged nitrogen 3 . The double bond is then hydrogenated, whereupon another equivalent of water is split off 6 . Due to a simultaneous electron transfer, the nitrogen is negatively charged after splitting off and can take up another proton 7 . During the hydrogenation and electron transfer, a hydroxide ion is absorbed by the iron and iron hydroxide [Fe (OH 2 ) or Fe (OH 3 )] is formed, which is finally converted into iron (II, III) oxide . The end product is aniline 8 .
Stoichiometry
- Nitroaromatics, iron and water react to aminoaromatics and iron (II, III) oxide.
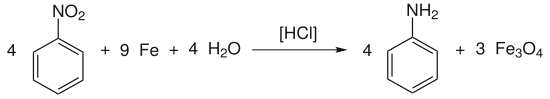
Example: Reduction of nitrobenzene to aniline
The given reaction equation reflects the overall process, which is built up into the following reaction steps:
The iron (II) chloride (FeCl 2 ) is continuously produced from the hydrochloric acid and iron.
Advantages and disadvantages
The main advantage is the low procedural costs. Iron is cheap, the resulting iron (II, III) oxide can be sold on as a color pigment and is therefore not to be regarded as waste, but as a product of value. The reaction requires precise adherence to carefully worked out reaction parameters, which has been achieved with the Laux process and is used on a large scale. This is usually easier to control in large-scale systems than on a laboratory scale. The exact elaboration of the procedure is also time-consuming. In the laboratory, therefore, reduction is often done catalytically or with other base metals such as zinc.
Another advantage of the method is that aromatics and double bonds are not hydrogenated.
On an industrial scale, however, catalytic hydrogenations are often used today to reduce nitroaromatics to anilines.
literature
- Organikum, 23rd edition, Wiley-VCH Verlag GmbH and Co. KGaA, 2000, pp. 633-636, ISBN 978-3-527-32292-3
- Zerong Wang: Comprehensive Organic Name Reactions and Reagents, Volume 1, Wiley, 2009, pp. 284-287, ISBN 978-0-471-70450-8
- Bradford P. Mundy, Michael G Ellerd, Frank G. Favaloro Jr .: Name Reactions and Reagents in Organic Syntheses, second Edition, Wiley-Interscience, 2005, p. 384 u. P. 285, ISBN 0-471-22854-0
Individual evidence
- ↑ a b Anthony R. Cartolano et al .: Amines by Reduction. In: Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley and Sons, New York 2004, 476-498.
- ^ Antoine Béchamp: De l'action des protosels de fer sur la nitronaphtaline et la nitrobenzine. Nouvelle method de formation des bases organiques artificielles de Zinin. In: Annales de chimie et de physique. 4, 1854, pp. 186-196 ( digitized on Gallica ).
- ↑ Kouichi Ohe, Sakae Uemura, Nobuyuki Sugita, Hideki Masuda, Toru Taga: The Journal of Organic Chemistry . tape 54 , 1989, pp. 4169-4174 , doi : 10.1021 / jo00278a034 .
- ↑ Alexander G. Kolchinski * † and Nathaniel W. Alcock ‡: The Journal of organic chemistry . tape 63 , 1998, pp. 4515-4517 , doi : 10.1021 / jo980057s .
- ^ Zerong Wang: Comprehensic Organic Name Reactions and Reagents , Volume 1, Wiley, 2009, p. 284, ISBN 978-0-471-70450-8 .
- ↑ PH Groggins: Unit Processes in Organic Synthesis . 5th ed. McGraw-Hill, New York, 1958, p. 143.
- ↑ Thomas Kahl, Kai-Wilfrid Schröder, FR Lawrence, WJ Marshall, Hartmut Höke, Rudolf Jäckh: Aniline. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, 2000, ISBN 3-527-30673-0 , doi : 10.1002 / 14356007.a02_303 .
- ↑ John J. McKetta: Nitrobenzene and Nitrotoluene . In: Encyclopedia of Chemical Processing and Design: Volume 31 - Natural Gas Liquids and Natural Gasoline to Offshore Process Piping: High Performance Alloys. CRC Press, 1989, ISBN 0-8247-2481-X .