1-benzylpiperazine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
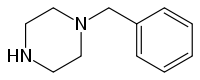
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-benzylpiperazine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 16 N 2 | |||||||||||||||
| Brief description |
light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 176.26 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.01 g cm −3 |
|||||||||||||||
| Melting point |
17-20 ° C |
|||||||||||||||
| Refractive index |
1.547 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1-Benzylpiperazine (abbreviation BZP ) was originally developed as an anti-parasitic agent . In animal experiments, however, antidepressant and amphetamine-like effects were discovered in rats and BZP was used as a drug in antidepressants and appetite suppressants . After severe side effects such as headache, high blood pressure up to vomiting and seizures occurred, the active ingredient was withdrawn from the market. BZP has a very stimulating and euphoric effect and is mainly consumed as a party drug . The effect is similar but weaker than the effect after taking amphetamine . As a drug, BZP is known under the names A2 , Frenzy or Nemesis . 1-Benzylpiperazine is freely available in most countries according to the current legal situation. However, in the opinion of the European Commission , it should be subject to drug control measures and criminal sanctions.
Legal situation (Germany and Europe)
Benzylpiperazine (BZP) is a synthetic substance that, like amphetamine and methamphetamine, stimulates the central nervous system. The Scientific Advisory Board of the European Monitoring Center for Drugs and Drug Addiction has, in accordance with Article 6 of Council Decision 2005/387 / JHA of 10 May 2005 on the exchange of information, risk assessment and control of new psychoactive substances (OJ L 127 of 20 May 2005 , Page 32) creates a risk assessment report. Thereafter, BZP should be monitored for its stimulant properties, health hazards and lack of medical benefits. As a result, the European Commission recommended that the Council of the European Union call on the Member States to take the necessary measures for the new synthetic drug BZP in accordance with their national legislation to ensure that BZP is subject to controls and criminal sanctions appropriate to the risks of the substance subject.
The Council decision (2008/206 / JI of March 3, 2008) became effective on March 8, 2008. Since the production, trade and consumption of BZP are associated with health and social risks and there is a risk situation, BZP in Germany should be subject to the Narcotics Act (BtMG) in anticipation of the Council decision . Due to its importance for research and analysis, the substance was included in Appendix II of the BtMG (marketable but not prescription narcotics) and is subject to the BtMG with effect from March 1, 2008 (cf. BGBl. I p. 246).
Individual evidence
- ↑ a b c d e f data sheet 1-Benzylpiperazine from Sigma-Aldrich , accessed on June 15, 2011 ( PDF ).
- ^ Erowid: History of BZP
- ↑ drug information pool: Benzylpiperazine
- ↑ Tagesschau: Strong warning against party drug BZP
- ↑ Decision 2008/206 / JI of the Council of March 3, 2008 on control measures and criminal law provisions for the new synthetic drug 1-benzylpiperazine (BZP) . In: Official Journal of the European Union . L 63, 2008, pp. 45-46.
- ↑ Twenty-first ordinance amending narcotics law , Federal Ministry of Justice and Consumer Protection, February 18, 2008.
