Bifunctional purine synthesis protein
| Bifunctional purine synthesis protein | ||
|---|---|---|

|
||
| Surface / band model of the PURH dimer according to PDB 1PKX . The AICAR formyltransferase domain is yellow and the IMP cyclohydrolase is rendered in blue. | ||
| Properties of human protein | ||
| Mass / length primary structure | 592 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene name | ATIC | |
| External IDs |
|
|
| Enzyme Classifications | ||
| EC, category | 2.1.2.3 , transferase | |
| Response type | Transfer of a formyl residue | |
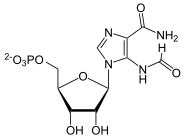
| Substrate | 10-formyl-THF + AICAR | |
| Products | THF + FAICAR | |
| EC, category | 3.5.4.10 , hydrolase | |
| Response type | Cyclizing dehydration | |
| Substrate | FAICAR | |
| Products | IMP + H 2 O | |
| Occurrence | ||
| Parent taxon | Creature | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 471 | 108147 |
| Ensemble | ENSG00000138363 | ENSMUSG00000026192 |
| UniProt | P31939 | Q9CWJ9 |
| Refseq (mRNA) | NM_004044 | NM_026195 |
| Refseq (protein) | NP_004035 | NP_080471 |
| Gene locus | Chr 2: 215.31 - 215.35 Mb | Chr 1: 71.56 - 71.58 Mb |
| PubMed search | 471 |
108147
|
The bifunctional Purinsyntheseprotein (ATIC, PURH) is the enzyme that, the last two steps in the de novo biosynthesis of inosine monophosphate (IMP), the formylation and subsequent dehydration of AICAR catalyzed . The individual enzyme functions are called AICAR formyltransferase and IMP cyclohydrolase . PURH occurs in all living things and the bifunctionality is preserved everywhere. Mutations in ATIC - gene of man can PURH deficiency and this AICA Ribosurie cause a rare inherited disorder.
PURH activity is increased by phosphorylation ; PURH is phosphorylated in several tumor cell lines .
Catalyzed reactions
AICAR is formylated to FAICAR.
FAICAR cyclizes with elimination of water to IMP.
The AICAR formyltransferase domain (amino acids 199-592) and the IMP cyclohydrolase domain (1-199) are 50 Å apart and retain their activity when expressed separately.
Individual evidence
- ↑ Orthologist at OMA
- ↑ UniProt P31939
- ↑ Boccalatte FE, Voena C, Riganti C, et al. : The enzymatic activity of 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase / IMP cyclohydrolase is enhanced by NPM-ALK: new insights in ALK-mediated pathogenesis and the treatment of ALCL . In: Blood . 113, No. 12, March 2009, pp. 2776-90. doi : 10.1182 / blood-2008-06-161018 . PMID 18845790 . PMC 2661863 (free full text).
- ↑ Cheong CG, Wolan DW, Greasley SE, Horton PA, Beardsley GP, Wilson IA: Crystal structures of human bifunctional enzyme aminoimidazole-4-carboxamide ribonucleotide transformylase / IMP cyclohydrolase in complex with potent sulfonyl-containing antifolates . In: J. Biol. Chem. . 279, No. 17, April 2004, pp. 18034-45. doi : 10.1074 / jbc.M313691200 . PMID 14966129 .
- ↑ Rayl EA, Moroson BA, Beardsley GP: The human pure gene product, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase / IMP cyclohydrolase. Cloning, sequencing, expression, purification, kinetic analysis, and domain mapping . In: J. Biol. Chem. . 271, No. 4, January 1996, pp. 2225-33. doi : 10.1074 / jbc.271.4.2225 . PMID 8567683 .
Web links
- D'Eustachio / reactome: 5'-phosphoribosyl-5-aminoimidazole-4-carboxamide (AICAR) + 10-formyltetrahydrofolate ⇒ 5'-phosphoribosyl-5-formaminoimidazole-4-carboxamide (FAICAR) + tetrahydrofolate
- Jassal / reactome: 5'-phosphoribosyl-5-formaminoimidazole-4-carboxamide (FAICAR) ⇔ inosine 5'-monophosphate + H2O