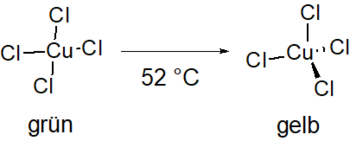Bis (diethylammonium) tetrachloridocuprate (II)
| This item has been on the quality assurance side of the editorial chemistry entered. This is done in order to bring the quality of the articles on the subject of chemistry in terms of form and content to the level desired in Wikipedia. We are grateful for your help , please take part in the discussion ( new entry ) or revise the article accordingly. |
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| Structural formula of the two ions of bis (diethylammonium) tetrachloridocuprate (II) in the high-temperature variant | |||||||
| General | |||||||
| Surname | Bis (diethylammonium) tetrachloridocuprate (II) | ||||||
| Molecular formula | C 8 H 24 Cl 4 CuN 2 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 353.65 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
77 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Bis (diethylammonium) tetrachloridocuprate (II) is a chemical compound and belongs to the group of cuprates . It is a thermochromic substance.
Extraction and presentation
Bis (diethylammonium) tetrachloridocuprate (II) is produced by the reaction of diethylammonium chloride with copper (II) chloride in an alcoholic solution.
properties
The green solid consists of needle-shaped crystals that are hygroscopic. It is readily soluble in ethanol and poorly in isopropanol . A phase transition of the first order takes place from a temperature of 52 ° C. The distorted square-planar cuprate ion transforms into the distorted tetrahedral form, which means that the absorption maximum shifts from the red to the purple range. The solid changes color from green to yellow.
The square-planar structure of the cuprate is stabilized with the help of hydrogen bonds ( ). This hydrogen bridge bond is weakened by thermal excitation and the conversion described takes place. The change in the strength of the hydrogen bonds can be detected with the help of an infrared (IR) spectrum , as there is a change in the absorption band in the range of the NH stretching vibration (approx. 3067 cm −1 ) . In addition, with an IR spectrum in the far IR range it can be shown that the coordination of the chloride ions on the Cu ion changes from square-planar to tetrahedral.
use
Due to its instability in relation to moisture and its specific temperature range, this thermochromic substance has no technical application. However, it can be used for teaching and exercise purposes, as it is uncomplicated to manufacture and can be examined with common laboratory methods such as an IR spectrometer or a UV / Vis spectrometer .
literature
- Darrell R. Bloomquist, Mark R. Pressprich, Roger D. Willett: Thermochromism in copper (II) halide salts. 4. Bis (diethylammonium) tetrachlorocuprate (II), structure of the high-temperature phase and physical characterization of its two phases . In: Journal of the American Chemical Society . tape 110 , no. October 22 , 1988, p. 7391-7398 , doi : 10.1021 / ja00230a020 .
- Sofiane Bouacida, Rafika Bouchene, Amina Khadri, Ratiba Belhouas, Hocine Merazig: Bis [4- (dimethylamino) pyridinium] tetrachloridocuprate (II) . In: Acta Crystallographica Section E: Structure Reports Online . tape 69 , Pt 11, 2013, ISSN 1600-5368 , p. m610 – m611 , doi : 10.1107 / S1600536813028006 , PMID 24454040 , PMC 3884264 (free full text).
- Christoph Janiak, Hans-Jürgen Meyer, Dietrich Gudat, Philipp Kurz: Riedel Modern Inorganic Chemistry . 5th edition. Walter de Gruyter, Berlin / Boston 2018, ISBN 978-3-11-044163-5 , pp. 427 ( limited preview in Google Book search).
Individual evidence
- ↑ a b c Sunhee Choi, James A. Larrabee: Thermochromic tetrachlorocuprate (II). An advanced integrated laboratory experiment . In: Journal of Chemical Education . No. 66 , September 1989, pp. 774-776 , doi : 10.1021 / ed066p774 ( wisc.edu ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Michiel JM Van Oort: Preparation of a Simple Thermochromic Solid . In: Journal of Chemical Education . No. 65 , January 1988, p. 84 , doi : 10.1021 / ed065p84 ( wisc.edu ).
![{\ displaystyle \ mathrm {2 [(C_ {2} H_ {5}) _ {2} NH_ {2}] Cl + CuCl_ {2} \ times 2H_ {2} O \ longrightarrow [(C_ {2} H_ { 5}) _ {2} NH_ {2}] _ {2} CuCl_ {4} + 2H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4000a3f3501c48987036c354334596b99168e5ca)