Lead (II) hexafluorosilicate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
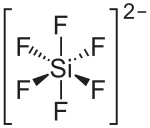
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Lead (II) hexafluorosilicate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Pb [SiF 6 ] | |||||||||||||||
| Brief description |
colorless to white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 349.27 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
very easily soluble in water (dihydrate: 1900 g l −1 at 0 ° C, 2220 g l −1 at 20 ° C, 4630 g l −1 at 100 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lead (II) hexafluorosilicate is a chemical compound of lead from the group of hexafluorosilicates , which is present as a dihydrate .
properties
As a dihydrate, lead (II) hexafluorosilicate is a colorless solid that is readily soluble in water. It decomposes when heated.
Extraction and presentation
Lead hexafluorosilicate can be obtained by reacting lead (II) oxide and hexafluoridosilicic acid.
use
Lead (II) hexafluorosilicate was previously used to renovate natural stone plasters. Today it is still used to remove lead through electrolysis . This method is also used to separate lead and bismuth by the Betts process .
Individual evidence
- ↑ a b c Entry on lead hexafluorosilicate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c Dale L. Perry: Handbook of Inorganic Compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , pp. 12, 215 ( limited preview in Google Book search).
- ↑ Entry on lead hexafluorosilicate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, 2015, ISBN 978-1-4822-6097-7 , pp. 4–71 ( limited preview in Google Book search).
- ↑ Christian Brandes: Paints and coatings for buildings made of natural stone Properties, requirements and practical application; with 14 tables . expert verlag, 1999, ISBN 978-3-8169-1536-2 , p. 28 ( limited preview in Google Book search).
- ↑ Facade protection and building renovation The guide for the renovation, conservation and restoration of buildings . expert verlag, 1994, ISBN 978-3-8169-0690-2 , p. 236 ( limited preview in Google Book search).
- ^ Mordechay Schlesinger, Milan Paunovic: Modern Electroplating . John Wiley & Sons, 2011, ISBN 1-118-06314-7 , pp. 250 ( limited preview in Google Book Search).
- ↑ Roger Rumbu: Non-Ferrous Extractive Metallurgy - Industrial practices . ISBN 1-312-59700-3 , pp. 252 ( limited preview in Google Book search).



![{\ displaystyle \ mathrm {PbO + H_ {2} [SiF_ {6}] \ longrightarrow Pb [SiF_ {6}] + H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/43a7d30ed14cbf191dd83f0264ccd4a85720c494)
