Brivaracetam
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
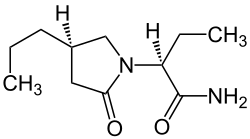
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Brivaracetam | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 11 H 20 N 2 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Ligand on synaptic vesicle protein 2A |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 212.29 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Brivaracetam is an antiepileptic effective drug . It was developed by the Belgian company UCB and approved in January 2016 in the EU and in February 2016 in the USA under the name Briviact for the additional treatment of focal epilepsy attacks in adults and adolescents aged 16 and over. Focal seizures are seizures that start on one side of the brain. If spread, they can affect larger areas on either side of the brain ("secondary generalization").
pharmacology
Mechanism of action
Brivaracetam is a ligand on synaptic vesicle protein 2A ( SV2A ). In addition inhibits it voltage-dependent sodium channels in the nervous system . The exact role of SV2A is not yet fully understood. Chemically, brivaracetam is derived from levetiracetam , with its higher affinity for the SV2A receptor having a stronger anticonvulsant activity in animal models.
Pharmacokinetics
Brivaracetam is almost completely absorbed in the gastrointestinal tract and excreted via the kidneys . The plasma half-life is 9 hours. Brivacetam has a low potential for drug interaction.
Early benefit assessment
In Germany, since 2011, newly approved drugs with new active ingredients must be subjected to an " early benefit assessment " by the Federal Joint Committee (G-BA) in accordance with Section 35a SGB V if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. The dossier evaluations, on the basis of which the G-BA makes its decisions, are created by the Institute for Quality and Efficiency in Health Care (IQWiG) .
Brivaracetam underwent two early benefit assessments due to an extension of approval. In 2016, the focus was on the additional treatment of partial seizures with or without secondary generalization in adults and adolescents aged 16 and over. In 2018 there was an assessment for children and young people from 4 to under 16 years of age. In both cases, the G-BA came to the conclusion that an additional benefit compared to the respective ACT is not proven.
literature
- P. von Rosenstiel: Brivaracetam (UCB 34714) . In: Neurotherapeutics . 4, No. 1, January 2007, pp. 84-87. doi : 10.1016 / j.nurt.2006.11.004 . PMID 17199019 .
- M. Bialer, SI.Johannessen, HJ.Kupferberg, RH.Levy, E. Perucca, T. Tomson: Progress report on new antiepileptic drugs: A summary of the Eight Eilat Conference (EILAT VIII) . In: Epilepsy Research . 73, No. 1, January 2007, pp. 5-6. doi : 10.1016 / j.eplepsyres.2006.10.008 . PMID 17158031 .
- A. Matagne, Margineanu, B. Kenda, P. Michel, H. Klitgaard: Anti-convulsiv and anti-epileptic properties of Brivaracetam (UCB34714), a high-affinity ligand for the synaptic vesicle protein, SV2A . In: British Journal of Pharmacology . 154, No. 8, August 2008, pp. 1662-1671. doi : 10.1038 / bjp.2008.198 . PMID 18500360 .
- DGA Kasteleijn-Nolst Trenité, P.Genton, D.Parain, P.Masnou, BJ.Steinhoff, T.Jacobs, E.Pigeolet: Evaluation of Brivaracetam, a novel SV2A Ligand in the photosensitivity model . In: Neurology . 69, No. 10, September 2007, pp. 1027-1034. doi : 10.1212 / 01.wnl.0000271385.85302.55 . PMID 17785672 .
- Doctors newspaper: Brivaracetam, New option for adults with focal epilepsy . In: Doctors newspaper . February 2016.
- Market Access & Health Policy: Market launch of "Briviact" in Germany . In: "market access & health policy" . February 2016.
Web links
Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Brivaracetam
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of (S) -2 - ((R) -2-oxo-4-propylpyrrolidin-1-yl) butanamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on 28 December 2019.
- ↑ Michael Freissmuth, Stefan Böhm, Stefan Offermanns: Pharmakologie und Toxikologie: From the molecular bases for pharmacotherapy 2012, p. 308.
- ↑ A16-08 Brivaracetam - Benefit assessment according to Section 35a SGB V; Accessed March 26, 2020.
- ↑ A16-38 Brivaracetam - Addendum to Commission A16-08; Accessed March 26, 2020.
- ↑ A18-48 Brivaracetam (epilepsy) - Benefit assessment according to Section 35a SGB V; Accessed March 26, 2020.
- ↑ Benefit assessment procedure for the active ingredient brivaracetam (focal seizures in epilepsy, ≥ 16 years); Accessed March 26, 2020.
- ↑ Benefit assessment procedure for the active ingredient brivaracetam (new area of application: focal seizures in epilepsy, additional therapy, 4 to <16 years); Accessed March 26, 2020.