Levetiracetam
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
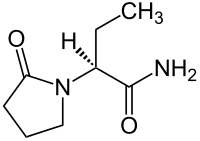
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Levetiracetam | ||||||||||||||||||
| other names |
( S ) -2- (2-Oxopyrrolidin-1-yl) -butylamide |
||||||||||||||||||
| Molecular formula | C 8 H 14 N 2 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 170.21 g · mol -1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Levetiracetam one is ethyl - derivative of dementia drug (former name: nootropic ) Piracetam . It is a drug from the group of anti-epileptics , which was developed by the Belgian company UCB SA and patented in 1985. It is available worldwide under the trade name Keppra ® . It has also been available as a generic since March 2011 .
It is not metabolized in the liver and is hardly bound to plasma proteins.
Admission
Levetiracetam may be prescribed as monotherapy for partial seizures with or without secondary generalization from the age of 16. In additional medication, this substance can be given for partial seizures from the age of one month. In addition, juvenile myoclonic epilepsy ( Janz syndrome ) from the age of 12 and primarily generalized tonic-clonic seizures in the context of idiopathic generalized epilepsy have been approved as additional therapy.
metabolism
The substance is hardly metabolized in the liver. There is one major inactive metabolite that is formed by hydrolysis in tissue.
Due to the lack of liver metabolism, the substance can be combined with all other anti-epileptic drugs without these influencing one another.
dosage
The recommended dose is divided into two daily doses. An increase is possible every two weeks. The dose should be reduced in patients with reduced kidney function (especially in the elderly).
effect
The mechanism of action is based on the binding to the vesicle protein SV2A , which results in a reduced release of glutamate from the presynaptic vesicle .
In vitro studies show that levetiracetam influences intra neuronal Ca2 + levels by partially inhibiting the Ca2 + current mediated by N-type channels and by reducing the release of Ca2 + from intraneuronal stores. It also partially reverses the reduction in GABA- and glycine -controlled currents induced by zinc and β-carbolines .
Side effects
The active ingredient levetiracetam is well tolerated. As with all anti-epileptic drugs, sudden discontinuation of the drug can provoke epileptic fits. Therefore, it has to be tapered off by slowly reducing the dose .
Side effects observed very commonly (≥10%):
- Asthenia ( feeling weak) and dizziness
- Somnolence (sleepiness)
Side effects that were observed frequently (1 to ≤10%) (excerpt):
Abdominal pain , agitation , anorexia , ataxia , dizziness , depression , diarrhea , diplopia , dyspepsia , eczema , emotional lability / mood swings , vomiting , rash , hostility / aggression , weight loss , weight gain, insomnia , itching , convulsion , headache , myalgia , nauseale amnesia , Nervousness / irritability , personality disorder , thrombocytopenia , tremor , increased cough , dizziness
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Levetiracetam
Individual evidence
- ↑ a b sheet levetiracetam at Sigma-Aldrich , accessed on 7 April 2011 ( PDF ).
- ↑ Entry on levetiracetam in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Klaus Aktories, Ulrich Förstermann, Franz Bernhard Hofmann, Klaus Starke (eds.): General and special pharmacology and toxicology . Founded by W. Forth, D. Henschler, W. Rummel. 2013, ISBN 978-3-437-16888-8 .
- ↑ Rote Liste Service GmbH (Ed.): Specialist information levetiracetam .
- ↑ Maren Boeck: Antiepileptics - Mechanisms of Action and Neuropsychological Side Effects. ( Memento from February 19, 2006 in the Internet Archive ).