Isobutene-isoprene rubber

Isobutene-isoprene rubber , or butyl rubber for short ( abbreviation IIR , sometimes also PIBI ), is a polymer from the group of synthetic rubbers . It is a copolymer of 95 to 99 mol% isobutene and 1–5 mol% isoprene . The vulcanizates of rubber have very low gas permeability and are very resistant to oxygen.
properties
Butyl rubber is often sold in yellowish-white, homogeneous bales of 30 kilograms. It dampens vibration and impact energy well and offers good resistance to acids and bases . Furthermore, it has very good weather and ozone resistance, a high electrical insulation capacity, very low gas permeability and elastic behavior even at very low temperatures. Disadvantages are its lack of resistance to oils and fats and its comparatively low elasticity at room temperature. The isoprene content determines the number of double bonds that are still present after the polymerization . These can be used for vulcanization or modification with halogens (chlorobutyl, bromobutyl). This allows various material parameters to be set:
- Hardness 40-85 Shore-A
- Tensile strength up to 20 N / mm 2
- Elongation at break up to 700%
- Operating temperature −40 ° C to +130 ° C
- Glass transition temperature T g = -73 ° C
Butyl rubber (IIR) must not be confused with the thermoplastic polyisobutylene (PIB).
History and manufacture
Butyl rubber was patented by Standard Oil of New Jersey (now Exxon ) in the USA in 1937 and developed by William J. Sparks and Robert M. Thomas , based on the previous development of polyisobutylene (PIB) at BASF in Germany (1931, Oppanol). It was widely used in the US military for inner tubes from 1943 onwards. In the 1950s and 1960s, halogenated butyl rubber (halobutyl) was further developed.
Since the cationic chain polymerization of isobutene in the presence of isoprene only gives sufficiently high molar masses at low temperatures , it is manufactured at low temperatures (−40 ° C to −100 ° C) in solution in n-hexane or by precipitation processes with the solvent dichloromethane instead of; acidic catalysts are aluminum (III) chloride or boron trifluoride .
application
Butyl rubber is used in air hoses, tire liners, heating bellows, seals and membranes , rubberized cable insulation, as chemical protection gloves, as corrosion protection for bridge ropes and as a component of chewing gum. Butyl rubber is also used for sealing thermal insulation glass and in adhesive and various heterogeneous solid rocket propellants .
Halobutyl rubber
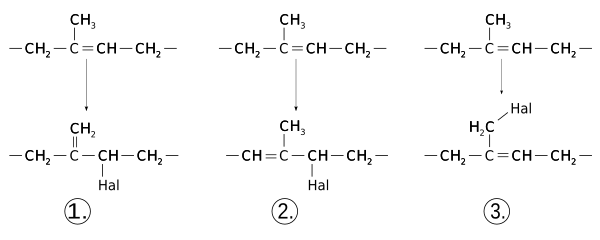
Butyl rubber can be improved in its properties by halogenation . To do this, the rubber is dissolved in an inert solvent and chlorine gas or liquid bromine is added while stirring vigorously. The resulting hydrogen halides are neutralized with sodium hydroxide solution.
Web links
Individual evidence
- ↑ Entry on butyl rubbers. In: Römpp Online . Georg Thieme Verlag, accessed on February 6, 2012.
- ^ Wissenschaft-Online-Lexika: Entry on "Rubber" in the Lexikon der Chemie, accessed on February 6, 2012.
- ↑ Rainer Saul, Oswald Vorteilel: Wrapping with butyl rubber tapes - an innovative corrosion protection for fully locked bridge ropes
- ↑ Sina Ebnesajjad: Adhesives Technology Handbook. 2nd Edition. William Andrew 2008, ISBN 978-0-8155-1533-3 , pp. 69-72.
- ↑ Jared Ledgard: The Preparatory Manual of Black Powder and Pyrotechnics. V1.4, Jared Ledgard 2007, ISBN 978-0-615-17427-3 , pp. 39, 51-52, 73, 77, 540, 549.
- ↑ LANXESS: What is butyl rubber? ( Memento from November 29, 2014 in the Internet Archive )