Calcium phosphite
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
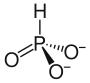
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Calcium phosphite | ||||||||||||||||||
| other names |
Calcium phosphonate |
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.06 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
> 300 ° C (decomposition) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Calcium phosphite , also called calcium phosphonate , is a chemical compound from the group of phosphonates . It usually occurs as the monohydrate CaH (PO 3 ) • H 2 O. This is when heated above 200 ° C crystal water from.
Extraction and presentation
Calcium phosphite can be obtained by reacting phosphonic acid with calcium oxide .
It is also formed as a by-product in the production of calcium hypophosphite Ca (H 2 PO 2 ) 2 .
use
Calcium phosphite is used as a fertilizer and as a polymerization catalyst .
swell
- ↑ a b Dale L. Perry, Sidney L. Phillips (Eds.): Handbook of Inorganic Compounds. CRC Press, Boca Raton FL et al. 1995, ISBN 0-8493-8671-3 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of calcium hydrogen phosphonate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on April 17, 2018, is reproduced from a self-classification by the distributor .
