Gravity Daniell Element
The Gravity-Daniell-Element describes a group of different further developments of the Daniell-Element developed in 1836 , a historical galvanic cell . Gravity Daniell elements were used as energy storage, among other things in electrical telegraphy until the beginning of the 20th century, they are among the so-called wet batteries .
construction
Like the Daniell element, all Gravity Daniell elements consist of two half-cells with a negative electrode made of zinc in a zinc sulfate solution and a positive electrode made of copper in a copper sulfate solution . In the original Daniell element, the two sulfalt solutions are separated by a diaphragm , also known as the salt conductor, which serves as an ion conductor and is made of earthenware (clay). Because of this diaphragm, the internal resistance of a Daniell cell is around 10 Ω , which at an element voltage of 1.1 V allows a maximum discharge current in the range of 100 mA , which is too little for many applications.
To reduce the internal resistance of the Daniell element, the high-resistance diaphragm was omitted and the two sulfate solutions brought into direct contact. In order to prevent undesired mixing, which would lead to failure of the element, the different densities of the two sulfate solutions are used for separation, which is where the term gravity for gravitation comes from. In the upper area of the Gravity-Daniell elements there is the lighter zinc sulphate solution with a zinc electrode , which can be designed as a ring or a grid, underneath the denser copper sulphate solution with a copper electrode. This type of galvanic element had a significantly lower internal resistance in the region of 1 Ω.
However, these cells are sensitive to vibrations, can only be operated in a vertical position and cannot be transported during operation, which means that mobile applications are excluded. In addition, if the cells are stored for a longer period of time, the lack of a diaphragm leads to increased diffusion of the two sulfate solutions and to self-discharge . Gravity cells were therefore put together immediately before commissioning and the beaker was slowly filled with the sulfate solutions in order to avoid mixing.
Designs
In total there are several different designs of Gravity-Daniell-Element which are named after their developers.
Meidinger element
The Meidinger element, named after the physicist Heinrich Meidinger , consists in the lower area of a glass beaker which is filled with copper sulphate solution, labeled with dd in the drawing, which shows the inside glass tube h and the insulated copper electrode inserted in the lower area encloses. The central glass tube inside contains a supply of solid copper sulphate, which can pass into the copper sulphate solution through a small opening in the lower area. The area above, labeled AA , is filled with the lighter zinc sulfate, with the zinc electrode in the upper area. The glass beaker in the lower area also has the task of delaying the mixing of the two solutions for as long as possible.
The Meidinger element was built in different shapes, as shown in the illustration on the right.
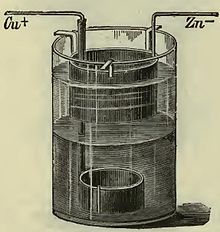
Callaud element
Due to the relatively complex glass structure of the Meidinger element, the Callaud element was developed around 1860, as shown above, which was used for decades in the telegraph sector due to its simplicity. This simplest form of a gravity cell consists only of a cup in which the two sulfate solutions are placed on top of each other. The copper electrode is in the lower area, sometimes with solid copper sulfate crystals in the bottom area for a higher amount of charge, the zinc electrode is in the upper area and is hung on the edge of the cup.
Lockwood element
The Lockwood element, which is similar to the Callaud element, represents an improvement in the electrode shape with increased capacity. The zinc electrode on top is made of solid zinc, in the lower area the spiral-shaped copper electrode is embedded in a reservoir made of copper sulfate.