Canagliflozin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
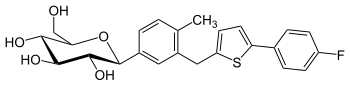
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Canagliflozin | ||||||||||||||||||
| other names |
(2 S , 3 R , 4 R , 5 S , 6 R ) -2- (3 - {[5- (4-fluorophenyl) thiophen-2-yl] methyl} -4-methylphenyl) -6- (hydroxymethyl) oxane-3,4,5-triol |
||||||||||||||||||
| Molecular formula | C 24 H 25 FO 5 S. | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 444.52 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Canagliflozin is an oral anti-diabetic drug used to treat type 2 diabetes mellitus . It was developed by Janssen-Cilag International NV .
pharmacology
Mechanism of action
Canagliflozin is a selective SGLT-2 inhibitor . The glucose, which is filtered off by the glomerulus , is reabsorbed to 99%. The transport proteins SGLT1 and SGLT 2, which transport glucose together with sodium from the primary urine into the tubular cells in the proximal tubule , are involved in this . The driving force for this is the sodium concentration gradient, which is maintained by the Na + / K + -APTase . SGLT1 is a cotransporter with low capacity but high affinity, while SGLT2 has high capacity but low affinity. Hypoglycaemia rarely occurs during treatment with canagliflozin , as SGLT2 only reaches 50% of its capacity under euglycaemic conditions. Under eu- and hypoglycemic conditions, SGLT1 has a higher capacity than SGLT2 and can thus compensate for inhibition of SGLT2.
Pharmacokinetics
Canagliflozin reaches maximum plasma levels 1–2 hours after oral intake, steady state plasma levels after 4–5 days. The bioavailability is 65%, the plasma protein binding 99%, whereby it is mainly bound to albumin . Canagliflozin only needs to be taken once a day as it lowers the renal threshold for glucose absorption for 24 hours. The half-life is 10.6 hours for a dose of 100 mg and 13.1 hours for a dose of 300 mg. Canagliflozin is mainly metabolized by O-glucuronidation via uridine diphosphate glucuronosyltransferase 1A9 (UGT1A9) and UGTB4. This creates inactive metabolites, 33% of which are eliminated renally and 42% are released via the faeces . It is only subject to a minimal cytochrome P450 metabolism, which means that it has little potential for interaction with other active substances.
Adverse drug effects
The most common adverse drug reactions with canagliflozin include both an increased risk of urinary tract infections and fungal infections in the genital area due to the high concentration of glucose in the urine. Headache, orthostatic hypotension , dizziness, increased urination and diarrhea are less common due to osmotic diuresis . A study published in the New England Journal of Medicine in June 2017 found evidence of a significantly increased risk of toe and metatarsal amputations compared to placebo .
Admission status
In November 2013, Canagliflozin received approval from the European Medicines Agency EMA under the name INVOKANA . The indication included patients with type 2 diabetes over the age of 18 years:
- as monotherapy in patients in whom a change in diet and physical activity are insufficient to lower blood sugar and who are not eligible for therapy with metformin
- As a complementary therapy with other antidiabetic drugs, if these, together with a change in diet and physical activity, are not sufficient to lower blood sugar.
Early benefit assessment
In Germany, since 2011, newly approved drugs with new active ingredients must be subjected to an " early benefit assessment " by the Federal Joint Committee (G-BA) in accordance with Section 35a SGB V if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. The dossier evaluations, on the basis of which the G-BA makes its decisions, are created by the Institute for Quality and Efficiency in Health Care (IQWiG) .
In September 2014, it was announced that Janssen-Cilag was discontinuing sales of INVOKANA in Germany. The reason for this is poor performance in an early benefit assessment by IQWiG. INVOKANA showed no additional benefit compared to the standard therapy with glimepiride plus metformin . The G-BA agreed with this assessment. Due to this assessment, Janssen-Cilag had to reckon with problems in negotiating prices with the health insurance companies and decided to take INVOKANA off the market. The G-BA was sharply criticized for this decision by the German Diabetes Society , since with this decision the G-BA prevented the introduction of new, more effective drugs with a better safety profile.
After the G-BA in 2015, based on a corresponding IQWiG dossier assessment, also found no additional benefit for the fixed combination of canagliflozin and metformin with the trade name VOKANAMET, Janssen-Cilag announced that it would also take this drug off the German market.
Recent study
In a CREDENCE study, the progression of chronic kidney disease in diabetics was significantly slowed down using canagliflozin. Nephrologists speak of a milestone. In addition to standard therapy (RAAS blockade with ACE inhibitors), it can significantly slow the progression of chronic kidney disease (CKD).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ T. Nagata, M. Fukazawa, K. Honda, T. Yata, M. Kawai, M. Yamane, N. Murao, K. Yamaguchi, M. Kato, T. Mitsui, Y. Suzuki, S. Ikeda, Y Kawabe: Selective SGLT2 inhibition by tofogliflozin reduces renal glucose reabsorption under hyperglycemic but not under hypo- or euglycemic conditions in rats. In: Am J Physiol Endocrinol Metab . 304, 2013, pp. 414-423, doi: 10.1152 / ajpendo.00545.2012 . PMID 23249697 .
- ↑ LH Opie: Sodium glucose co-transporter 2 (SGLT2) inhibitors: new among antidiabetic drugs. In: Cardiovasc Drugs Ther . 28, 2014, pp. 331–334, doi: 10.1007 / s10557-014-6522-0 . PMID 24825435 .
- ^ E. Dietrich, J. Powell, JR Taylor: Canagliflozin: a novel treatment option for type 2 diabetes. In: Drug Des Devel Ther . 7, 2013, pp. 1399-1408, doi: 10.2147 / DDDT.S48937 . PMID 24285921 .
- ^ AK Niazi, SH Niazi: A novel strategy for the treatment of diabetes mellitus - sodium glucose cotransport inhibitors In: N Am J Med Sci . 2, 2010, pp. 556-560, doi: 10.4297 / najms.2010.2556 . PMID 22558567 .
- ↑ M. Pfister, JM Whaley, L. Zhang, JF List: Inhibition of SGLT2: a novel strategy for treatment of type 2 diabetes mellitus In: Clin Pharmacol Ther . 89, 2011, pp. 621-625, doi: 10.1038 / clpt.2011.16 . PMID 21346749 .
- ↑ Bruce Neal, Vlado Perkovic, Kenneth W. Mahaffey, Dick de Zeeuw, Greg Fulcher: Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes . In: New England Journal of Medicine . June 12, 2017, doi : 10.1056 / nejmoa1611925 .
- ↑ Clinical Trial Results Find Increased Risk of Leg and Foot Amputations
- ↑ Product information Invokana. European Commission's Directorate for public health and risk assessment.
- ↑ A14-12 Canagliflozin - Benefit assessment according to Section 35a SGB V (dossier assessment); Accessed March 26, 2020.
- ↑ A14-24 Addendum to Commission A14-12 (Canagliflozin); Accessed March 26, 2020.
- ↑ Benefit assessment procedure for the active ingredient canagliflozin (diabetes mellitus type 2); Accessed March 26, 2020.
- ↑ Sales stop : No canagliflozin for German diabetics. In: Pharmaceutical newspaper online. September 26, 2014.
- ↑ A14-27 Canagliflozin / metformin - Benefit assessment according to Section 35a SGB V; Accessed March 26, 2020.
- ↑ Benefit assessment procedure for the active ingredient canagliflozin / metformin (type 2 diabetes mellitus); Accessed March 26, 2020.
- ^ According to G-BA decision: Janssen discontinues Vokanamet sales. In: Deutsche Apotheker Zeitung online. 17th February 2015.
- ↑ Vlado Perkovic, Meg J. Jardine a. a .: Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. In: New England Journal of Medicine. April 14, 2019, doi : 10.1056 / NEJMoa1811744 .