Cannabichromes
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
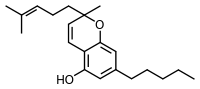
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cannabichromes | ||||||||||||||||||
| Molecular formula | C 21 H 30 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 314.47 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
144-146 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Cannabichromene (also CBC ) is one of 113 cannabinoids discovered so far . It does not have an analgesic effect itself, but it supports the pain-relieving effect of tetrahydrocannabinol (THC) and has a calming effect. In addition, an antibiotic effect was found against bacteria that are considered to be resistant to classic antibiotics . When cannabichromene is exposed to light, it can produce cannabicyclol . The occurrence of CBC is higher in cannabis indica than in cannabis sativa .
In the US , CBC is used in the form of medical cannabis , e.g. B. against epilepsy and to support the analgesic effect of tetrahydrocannabinol.
Individual evidence
- ↑ Entry on cannabinoids. In: Römpp Online . Georg Thieme Verlag, accessed on July 7, 2013.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Oier Aizpurua-Olaizola, Umut Soydaner, Ekin Öztürk, Daniele Schibano, Yilmaz Simsir: Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes . In: Journal of Natural Products . tape 79 , no. 2 , February 26, 2016, ISSN 0163-3864 , p. 324-331 , doi : 10.1021 / acs.jnatprod.5b00949 .
- ↑ Possible uses of cannabis sativa L. in medicine . GRIN Verlag, 2003, ISBN 3-638-21252-1 , p. 9 ( limited preview in Google Book search).
- ↑ CE Turner, MA ElSohly: Biological activity of cannabichromene, its homologs and isomers. In: Journal of clinical pharmacology. Volume 21, Numbers 8-9 Suppl, 1981 Aug-Sep, ISSN 0091-2700 , pp. 283S-291S, PMID 7298870 .