Citric acid trimethyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
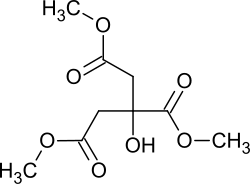
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Citric acid trimethyl ester | |||||||||||||||
| other names |
Trimethyl citrate |
|||||||||||||||
| Molecular formula | C 9 H 14 O 7 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 234.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
75-78 ° C |
|||||||||||||||
| boiling point |
283–287 ° C (partial decomposition) |
|||||||||||||||
| solubility |
very soluble in ethanol and diethyl ether |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Citric acid trimethyl ester is a chemical compound from the group of carboxylic acid esters .
Occurrence
Citric acid trimethyl ester is one of the components of Dioscorea opposita .
Extraction and presentation
Citric acid trimethyl ester can be obtained by reacting citric acid with methanol in the presence of hydrochloric acid .
properties
Citric acid trimethyl ester is a white crystalline solid that is very soluble in ethanol and diethyl ether.
use
Citric acid trimethyl ester can be used in deodorants to reduce odors. The compound is also used as a plasticizer for PVC .
Individual evidence
- ↑ a b c d e f g Data sheet Trimethyl citrate, ≥98.0% from Sigma-Aldrich , accessed on December 15, 2018 ( PDF ).
- ↑ A. Townshend, DT Burns, Ryszard Lobinski, EJ Newman, G. Guilbault, Z. Marczenko, H. Onishi: Dictionary of Analytical Reagents . CRC Press, 1993, ISBN 978-0-412-35150-1 , pp. 254 ( limited preview in Google Book search).
- ^ A b Chemical Society (Great Britain): Journal of the Chemical Society . The Society, 1877, pp. 456 ( limited preview in Google Book search).
- ↑ a b David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 554 ( limited preview in Google Book search).
- ↑ George Wypych: Handbook of Odors in Plastic Materials . William Andrew, 2013, ISBN 1-4557-2833-0 ( limited preview in Google Book Search).
- ↑ Google Patents: EP0006234A1 - Use of a combination of esters of citric acid and / or acetylcitric acid with antioxidants as deodorants - Google Patents , accessed December 15, 2018
- ↑ Guneri Akovali: Plastics, Rubber and Health . iSmithers Rapra Publishing, 2007, ISBN 978-1-84735-081-7 , pp. 51 ( limited preview in Google Book search).