Cycloheptanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
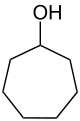
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cycloheptanol | |||||||||||||||
| other names |
Hydroxycycloheptane |
|||||||||||||||
| Molecular formula | C 7 H 14 O | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.96 g cm −3 |
|||||||||||||||
| Melting point |
7 ° C |
|||||||||||||||
| boiling point |
185 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.477 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cycloheptanol is a chemical compound from the group of cycloalkanols .
Extraction and presentation
Cycloheptanol can be obtained from aminomethylcyclohexane by ring expansion using the Demjanow rearrangement . It is also possible to prepare by reducing cycloheptanone with hydrogen .
properties
Cycloheptanol is a colorless oily flammable liquid that is practically insoluble in water.
safety instructions
The vapors of cycloheptanol can form an explosive mixture with air ( flash point 60 ° C).
Individual evidence
- ↑ a b c d e f g h Entry for CAS no. 502-41-0 in the GESTIS substance database of the IFA , accessed on May 2, 2015(JavaScript required) .
- ↑ a b William M. Haynes: CRC Handbook of Chemistry and Physics, 95th Edition: . CRC Press, 2014, ISBN 978-1-4822-0868-9 , pp. 3–113 ( limited preview in Google Book search).
- ↑ a b Data sheet Cycloheptanol, 97% from Sigma-Aldrich , accessed on May 2, 2015 ( PDF ).
- ↑ Eberhard Breitmaier, Günther Jung: Organic Chemistry, 7th complete revision. u. exp. Edition 2012: Basics, compound classes, reactions, concepts, molecular structure, natural substances, synthesis planning, sustainability . Georg Thieme Verlag, 2014, ISBN 978-3-13-159987-2 , p. 117 ( limited preview in Google Book search).
- ^ Robert J. Ouellette, J. David Rawn: Organic Chemistry: Structure, Mechanism, and Synthesis . Elsevier, 2014, ISBN 978-0-12-801082-2 , pp. 514 ( limited preview in Google Book search).
