Cyclotene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
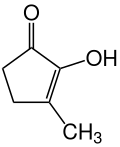
| Enol form | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cyclotene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 8 O 2 | ||||||||||||||||||
| Brief description |
Crystalline solid with a spicy, caramel-like smell |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 112.13 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
104-108 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Cycloten is an organic chemical compound with an intense caramel-like odor. Chemically, it is an enol and ketone and not a lactone , such as the professionally incorrect name Ahornlacton (Engl. Maple lactone ) suggests. Cycloten is a flavoring substance that occurs in various prepared foods and is used for flavoring in many areas.
history
The compound was isolated for the first time in 1912 by the chemist Julius Meyerfeld from the wood vinegar of the birch wood. He himself described the smell as singularly pleasant and the taste as sweet, somewhat burning, reminiscent of liquorice and fresh walnuts .
Origin and occurrence
Cycloten is formed, among other things, in the Maillard reaction of glucose . Therefore, it is found mainly in thermally processed foods and provides a caramel note in their aroma. Examples of such foods are coffee , bread, and roasted nuts . But it is also found in many maple products such as maple sugar and maple syrup .
It also occurs in birch wood tar .
Extraction and presentation
Cycloten is mainly obtained from birch tar by extraction . In addition, numerous different synthesis options are known, although synthetic production plays a subordinate role.
properties
Aroma properties
The aroma of cycloten is described as caramel-like, spicy and sweet. Sometimes a liquorice note is also perceived. In addition to its own flavor, it also enhances the sweetness of sugar .
Chemical properties
Cyclotene is normally in enol form 1 with one keto and one enol group. However, it is subject to the keto-enol tautomerism , so that, depending on the environmental conditions - especially in acidic solutions - the keto form 2 , which is a diketone , also occurs.
Theoretically, another enol form is conceivable if the other keto group of 2 enolizes, but this is not formed.
Cyclotene is often present as a hydrate with one mole of water of crystallization .
use
Cycloten is added to various foods such as sweets and beverages as a flavoring .
Individual evidence
- ↑ a b c d e f g h i Entry on 2-hydroxy-3-methyl-2-cyclopenten-1-one. In: Römpp Online . Georg Thieme Verlag, accessed on November 28, 2019.
- ↑ External identifiers or database links for 3-methylcyclopentane-1,2-dione : CAS number: 765-70-8, EC number: 212-154-8, ECHA InfoCard: 100.011.049 , PubChem : 61209 , ChemSpider : 55153 , Wikidata : Q72489740 .
- ↑ a b Data sheet Methyl cyclopentenolone anhydrous, 98%, FCC, FG from Sigma-Aldrich , accessed on November 28, 2019 ( PDF ).
- ↑ a b c d George M. Strunz: Synthetic Routes to 2-Hydroxy-3-methylcyclopent-2-en-l-one and Related Cyclopentane-1,2-diones: A Review . In: Journal of Agricultural and Food Chemistry . tape 31 , no. 2 , 1983, p. 185-190 .
- ↑ Patent US5856582 : Process for the manufacture of 2-hydroxy-3-methylcyclopent-2-ene-1-one. Published January 5, 1999 , inventor: Glen Francis Crum.
- ↑ a b c d e f g Jens Schrader: Microbial Flavor Production . In: Ralf Günter Berger (Ed.): Flavors and Fragrances - Chemistry, Bioprocessing and Sustainability . Springer, Berlin 2007, ISBN 978-3-540-49338-9 , 2.4.3.24, pp. 105 , doi : 10.1007 / b136889 .
- ↑ Patent US5661037 : Methods of detecting tautomeric cyclic 1,2-diones. Published August 26, 1997 , Inventors: Jian Steven Qi, David E. Albright & Garra C. Lester.


