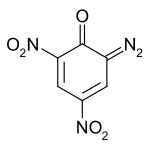
Diazodinitrophenol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diazodinitrophenol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 2 N 4 O 5 | |||||||||||||||
| Brief description |
yellowish to red-yellowish amorphous powder, but darkens quickly in sunlight |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 210.1 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.63 g cm −3 |
|||||||||||||||
| solubility |
Slightly soluble in water, somewhat soluble in methanol and ethanol , soluble in acetone , nitroglycerin , nitrobenzene , aniline , pyridine and acetic acid |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Diazodinitrophenol is a chemical compound that is used as an initial explosive .
presentation
Diazodinitrophenol is produced by diazotization from 2-amino-4,6-dinitrophenol .
properties
The connection is particularly explosive when dry due to impact, friction, heat and other ignition sources and is subject to the Explosives Act .
Table with important explosion-relevant properties: Oxygen balance −60.9% Nitrogen content 26.67% Normal gas volume 859 l kg −1 Explosion heat 3998.8 kJ kg −1 (H 2 O (l))
3967.5 kJ kg −1 (H 2 O (g))Specific energy 1047.9 kJ kg −1 (107.0 mt / kg) Lead block bulge 32.6 cm 3 g −1 Detonation velocity 6600 m s −1 Deflagration point 180-200 ° C Sensitivity to impact 1.5 Nm
use
The diazo compound is used as an initial explosive in the USA . It is stronger than fiery mercury and somewhat weaker than lead azide and was introduced in primers as a replacement for the lead and mercury-containing primers .
Individual evidence
- ↑ a b c d e f g h i j k l Köhler, J .; Meyer, R .; Homburg, A .: Explosivstoffe , tenth, completely revised edition, Wiley-VCH, Weinheim 2008, p. 89, ISBN 978-3-527-32009-7 .
- ↑ a b Registration dossier on 6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one ( GHS section ) at the European Chemicals Agency (ECHA), accessed on January 15, 2020.
- ↑ LV Clark: "Diazodinitrophenol, a Detonating Explosive", in: Ind. Eng. Chem. 1933 , 25 (6) , pp. 663-669; doi : 10.1021 / ie50282a021 .
- ↑ Manufacture of diazodinitrophenol
- ↑ Roth, L .; Weller, U .: Hazardous Chemical Reactions , 65th supplement, ecomed-Verlag 2011.
- ↑ Explosives Act, Appendix I, List of Explosive Substances ( BGBl. 1975 I p. 853 ), to which the law is to be applied in full.
literature
- Jared Ledgard: The Preparatory Manual of Explosives. Lulu.com, 2007, ISBN 978-0-615-14290-6 , p. 141 ( limited preview in Google book search).
- J. Köhler, Rudolf Meyer, Axel Homburg, Explosivstoffe, ISBN 978-3-527-32009-7 , 10th edition, pp. 89f.
