Dichlorohexoxide
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Dichlorohexoxide | ||||||
| other names |
Chloryl perchlorate |
||||||
| Molecular formula | Cl 2 O 6 | ||||||
| Brief description |
deep red liquid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 166.90 g mol −1 | ||||||
| Physical state |
liquid |
||||||
| density |
2.02 g cm −3 |
||||||
| Melting point |
3.5 ° C |
||||||
| boiling point |
203 ° C (theoretical) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Dichlorohexaoxide is a chemical compound from the group of chlorine oxides .
Extraction and presentation
Dichlorohexaoxide can be obtained by reacting chlorine dioxide with ozone .
It is also possible to display it by reacting chlororyl fluoride and perchloric acid .
properties
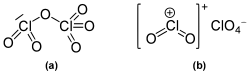
Dichlorohexaoxide is a deep red liquid that can be stored undecomposed below −30 ° C. It is the least explosive of all chlorine oxides, but it explodes on contact with organic matter. As a gas, it largely dissociates to form chlorine trioxide, which breaks down into chlorine dioxide and oxygen or into chlorine and oxygen at room temperature.
In the gas phase and as a liquid, it is present as covalent chlororyl perchlorate O 2 Cl-O-ClO 3 . The oxidation states of chlorine are here V and VII. In the solid, the compound exists as chlororyl chlorate with isolated ClO 2 + and ClO 4 - ions.
The compound is the mixed anhydride of chloric and perchloric acid. When reacting with water chloric acid and perchloric acid are formed, with ozone slowly dichloroheptaoxide .
Individual evidence
- ↑ a b c d e f g Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 315.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d e Ralf Steudel : Chemistry of Non-Metals, Syntheses - Structures - Bonding - Use , 4th Edition, 2014 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-030439-8 , Pp. 553–554, (accessed via De Gruyter Online).
- ↑ Klaus M. Tobias, Martin Jansen: Structure of Cl 2 O 6 in the crystal. In: Angewandte Chemie 98, 1986, pp. 994-995, doi : 10.1002 / anie.19860981108 .


