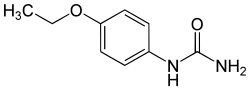
Dulcin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dulcin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 12 N 2 O 2 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 180.21 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
173 ° C |
||||||||||||||||||
| solubility |
1.25 g l −1 in water (25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dulcin ( p- phenetol carbamide) is a sweetener that is 200 times sweeter than sucrose . Dulcin is a derivative of urea and is similar to Suosan . Dulcin is not approved for use with food within the EU.
history
Dulcin was discovered by Joseph Berlinerblau in 1884 and was the second synthetic sweetener after saccharin . Since the first medical tests showed that the sweetener was harmless to the human organism, it was launched in the USA in 1891.
Several studies later found that dulcin caused cancer of the liver and bladder in animal experiments with rats . As a result of these results, the sweetener was withdrawn from FDA approval in 1950 .
The Expert Panel of the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), the Joint FAO / WHO Expert Committee on Food Additives (JECFA), decided in 1976 that dulcin should not be used as a feed additive.
Chemical properties
Dulcin decomposes in the heat in water and hydrolyzes in a 0.1 N solution of acetic acid.
Individual evidence
- ↑ Entry on Dulcin. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ^ A b Fritz Ullmann: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH; 6th edition 2003; ISBN 3-527-30385-5 ; P. 425.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Lyn O'Brien-Nabors (Ed.): Alternative Sweeteners . Marcel Dekker Inc., 3rd ed. 2001. ISBN 0-8247-0437-1 ; P. 222f.
- ↑ K. Taufel, B. Flemm: In studies on natural and artificial sweeteners. I. Studies on the degree of sweetening of saccharin and dulcin, investigation of Nahr. Luxury foods , 1925 , 50 , pp. 264-273.
- ^ RH Goldsmith, Dulcin: A Centennial Perspective, In Journal of Forensic Sciences , 1986 , 31 , p. 1.
- ^ Joint FAO / WHO Expert Committee on Food Additives (JECFA), Monograph für Ethoxyphenylurea, 4- (Dulcin) , accessed December 9, 2014.
- ↑ FDA, PART 189 - SUBSTANCES PROHIBITED FROM USE IN HUMAN FOOD
- ↑ Andrew Wallace Hayes, Principles and Methods of Toxicology, CRC Press, 2001 , p. 1887, ISBN 1-56032-814-2 .
- ^ Joint FAO / WHO Expert Committee on Food Additives (JECFA), Evaluation for 4-ETHOXYPHENYLUREA , accessed on December 9, 2014.