Real Time Quantitative PCR
The quantitative real-time PCR or engl. real-time quantitative PCR ( qPCR for short or Real Time Detection PCR , RTD-PCR for short ) is a replication method for nucleic acids that is based on the principle of the conventional polymerase chain reaction (PCR) and also enables the DNA obtained to be quantified . The qPCR is sometimes ambiguously referred to for reverse transcription with subsequent q-PCR (in the same approach) as qRT-PCR or RT-qPCR .
Quantification is by means of fluorescence conducted measurements, during a PCR cycle in real time (engl. Real time ) are recorded. The fluorescence increases proportionally with the amount of PCR products. At the end of a run (which consists of several cycles), the quantification is carried out in the exponential phase of the PCR on the basis of the fluorescence signals obtained. Correct quantification is only possible in the exponential phase of the PCR (which lasts a few cycles in one run), since the optimal reaction conditions prevail during this phase. This method differs from other quantitative PCR methods (qPCR), which only carry out a quantitative evaluation (e.g. competitive PCR ) after the end of the PCR , usually including a gel electrophoretic separation of the PCR fragments.
Real-time quantitative PCR can also be used for other purposes, e.g. B. to differentiate between homozygous and heterozygous characteristics.
The abbreviation RT-PCR or qRT-PCR is sometimes used for qPCR. However, this leads to confusion, since this abbreviation is already used for the reverse transcriptase-polymerase chain reaction .
Methods
Dyes
The simplest way of quantifying the PCR products is to use DNA dyes (e.g. ethidium bromide or SYBR Green I ). These fluorescent dyes are deposited into the DNA of a (intercalate) and bind (to the minor groove of double stranded DNA English minor groove binder ), whereby the fluorescence increase of these dyes. The increase in the stained target DNA therefore correlates with the increase in fluorescence from cycle to cycle. The measurement takes place at the end of the elongation in each cycle.
A disadvantage of this method is the low specificity, since it is not possible to differentiate between different PCR products. In addition, multiplex measurements cannot be carried out. The first disadvantage can be compensated for by performing a melting curve analysis after the end of the PCR , on the basis of which the fragment length (s) and thus the specificity can be determined.
In a melting curve analysis , the DNA is melted by slowly increasing the temperature continuously (50 ° C → 95 ° C). At a specific melting temperature for the fragment, the double strand denatures to two single-stranded molecules. A fluorescent dye (e.g. SYBR Green I) is released and a change in fluorescence is registered. The melting curve and the melting temperature of a dsDNA fragment are essentially dependent on the length and base composition of the DNA fragment. Melting curve analysis can be used to obtain information about the specificity of the PCR reaction. Since the double-stranded DNA of specific PCR products has a higher melting point than unspecific primer dimers, a differentiation is possible. Another use of melting curve analysis is the comparison and differentiation of different PCR products (different DNA sequences, possibly different fragment sizes) with regard to their melting behavior. In many cases, the melting curve is displayed by plotting the absolute value of the first derivative of the fluorescence signal (y-axis) against the temperature (x-axis). The melting curve peaks thus represented are characteristic of a particular PCR product and the melting curve analysis is therefore used for a qualitative examination of the PCR product. The height (or any other feature) of the melting curve peaks is not a parameter for quantifying the amount of DNA.
FRET probes
Another possibility is to use the Förster resonance energy transfer (FRET). A donor fluorochrome (reporter - in connection with TaqMan probes), which is excited by a light source, gives off part of its energy to a sufficiently close acceptor fluorochrome (or a dark quencher - in connection with TaqMan probes) . If the distance between acceptor and donor increases, FRET and thus the fluorescence signal of the acceptor decrease, while that of the donor increases. This method is very complex and expensive, but offers the advantages of the high specificity of the assay .
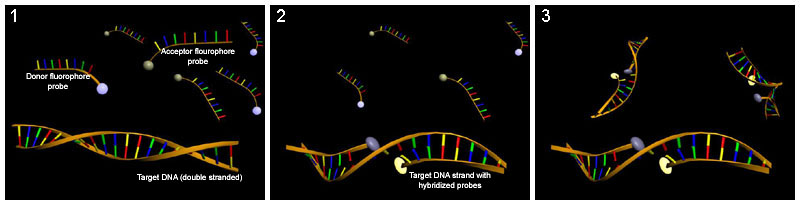
LightCycler probes (also hybridization probes)
The easiest way to use FRET to quantify nucleic acids is to use LightCycler probes. Two different oligonucleotides , each labeled with a FRET donor or FRET acceptor (here reporter) , which bind next to one another to the target sequence and thus bring the fluorochromes close enough for FRET, can be used as probes for the quantification of the PCR -Products are used. The measurement takes place at the end of the annealing phase in each cycle. A melting curve analysis can also follow here.
LoopTag probes
The FRET principle is also used in the LoopTag probe system. This system consists of the forward primer, at the 5 'end of which a sequence-unspecific nucleotide sequence and the fluorescence acceptor are coupled. The other part is the detection probe (LoopTag probe), to which a sequence-unspecific nucleotide sequence and the donor are coupled at the 3 'end. The 5 'end of the LoopTag probe hybridizes specifically to the target sequence and specifically to the extended forward primer at the 3' end. The sequence-unspecific nucleotide sequences of the detection probe and the forward primer hybridize with one another to form a probe-primer loop and form a stem. The resulting spatial proximity of the terminal dyes (donor / probe, acceptor / primer) enables the energy transfer to take place.
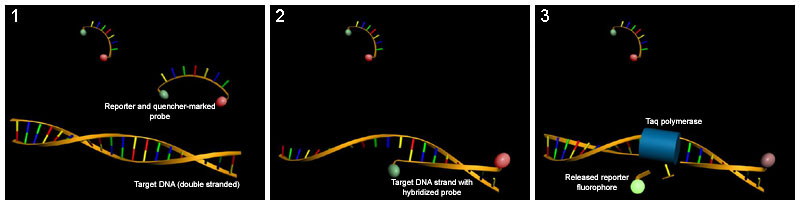
TaqMan probes (also hydrolysis probes)
Another frequently used option of FRET is to use a probe that has been labeled with a quencher at one end and with a reporter fluorescent dye (e.g. TAMRA and FAM ) ( double-dye oligos , TaqMan Probe ). If the Taq polymerase , which in addition to the polymerase activity has a 5'-3 ' exonuclease activity, degrades the probe during the synthesis of the opposite strand at the 5' end, the quencher and fluorophore move away from each other and the reporter fluorescence increases can be measured. The measurement takes place at the end of the elongation in each cycle.
Molecular Beacons
Another possibility of real-time quantification of PCR products using FRET is the use of molecular beacons as probes. Molecular beacons are oligonucleotides that are coupled to both a reporter fluorophore and a quencher. The nucleotides at the 5 'end of the probe are complementary to those at the 3' end, so that a secondary structure characteristic of molecular beacons can develop. In this state, known as stem loop (hairpin structure), the reporter shows no fluorescence due to its small distance from the quencher. By adding the loop region to a complementary DNA sequence during a PCR cycle, the distance between quencher and reporter is increased. Reporter fluorescence can thus be observed.
Scorpion primer
Scorpion primers are complex oligonucleotides which combine the properties of real-time PCR probes and PCR primers in one (Uni-Scorpion) or two molecules (Bi-Scorpion). Similar to Molecular Beacons, they have a characteristic secondary structure with a self-complementary shaft region, the ends of which have been modified with a reporter fluorophore and a quencher. In addition, these probes carry a PCR primer at the 3 'end. During a PCR cycle, with increasing DNA concentration, reporter fluorescence can be observed due to the attachment of the loop region to a complementary DNA sequence and thus increased distance between quencher and reporter.
Lux primer
Lux primers are oligonucleotides marked with a fluorescent dye, the fluorescence intensity of which depends on their respective chemical environment. If these primers are incorporated into a DNA during a PCR, an increase in fluorescence can be observed.
Lanthanoid-labeled probes
Lanthanide-labeled probes at their 5 'end with a lanthanide such as europium or terbium labeled and hybridized with an oligonucleotide, the 3' end is labeled with a quencher on.
quantification
Various calculation models are used for the quantification, with a reference gene (e.g. GAPDH , actin , tubulin ) usually also being measured in order to carry out a relative quantity comparison (relative quantification). Other, far more complicated methods are intended to enable absolute quantification, in which the exact number of templates present in the sample can be determined.
Ct value or also Cp value
In the first phase of the amplification of a PCR, the amount of template is limited and the probability that template, primer and polymerase will meet is suboptimal, while in the third phase of amplification the amount of products (DNA, pyrophosphate, monophosphate nucleotides) increases to such an extent that inhibited by this, product fragments hybridize more frequently with one another, the substrates are slowly consumed and ultimately the polymerases and nucleotides are slowly destroyed by the heat. An exponential and therefore quantifiable increase can only be found in the phase in between. A PCR remains exponentially with 12 to 400 initial copies for approx. 30 cycles, with 200 to 3200 for 25 cycles and with initially 3200 to 51200 for a maximum of 20 cycles. In order to always be able to measure at the beginning of the exponential phase, the Ct value ( cycle threshold for threshold value cycle) or the Cp value ( crossing point ) is often used, which describes the cycle at which the fluorescence occurs increases significantly above the background fluorescence for the first time.
Efficiency
The efficiency can be calculated in several ways, which differ slightly in their result. The simplest way is as follows:
The efficiency E can be calculated using the slope m of a standard curve. For this purpose, a cDNA dilution approach (e.g. 100%, 10%, 1%, 0.1%) is used for a qPCR, and the respective Ct values are used to graphically generate a curve. A linear regression line through the curve has the slope -m (when plotted with increasing DNA concentration).
A slope m of −3.32 would therefore mean an efficiency of 1 (100%), i.e. H. a doubling of the amplificates per cycle, a slope of −3.58 an efficiency of 0.9 (90%). The formula provides meaningful values below 100% only for slope values less than −3.32.
Absolute quantification
An absolute quantification is time-consuming and the results are questionable, which is why this type of quantification is rarely carried out. Among other things, the efficiency of reverse transcription , which can be between 5 and 95%, must be determined, e.g. B. by using synthesized RNA of known amount.
Relative quantification
An internal control is required for this. An internal control can be a gene transcript, the signal of which is used to compensate for variations in the initial amount of the RNA used. For this z. B. Household genes used. The comparison of quantities with the household genes is called normalization. Because the overall analysis is based on this signal, the choice of internal control is an important aspect of the experiment.
The ideal internal control is easy to detect and its expression should not vary during the cell cycle, between cell types, or in response to experimental treatment (e.g., stress, drugs, disease).
Calculation using a standard curve
There is a linear, inversely proportional relationship between the logarithm of the amount used and the Ct. If the initial amount is known, a standard curve can be constructed by plotting the logarithm of the initial amount against the Ct. By the straight line equation
- x = (Ct - b) / m
the logarithm of the copy number can be determined from the standard curve for each unknown sample. All samples are normalized by dividing the calculated number of copies of the target gene by the number of copies of the internal reference:
- Gene (normalized) = target copy number / reference copy number
The different expression of two samples relative to one another can be represented as a quotient and results in an n-fold expression:
- Gene (normalized) (group A) / gene (normalized) (group B) = n-fold expression group A to group B
Calculation according to the ΔΔCt method
The different expression is indicated as n-fold expression with the aid of the ΔΔCt value. In this process, it is important that the two PCR reactions involved are equally efficient. First, the Ct values of the target gene and reference are subtracted from one another (ΔCt) in order to then form the difference between the two ΔCt values of the individual groups (e.g. sick / healthy, with / without active ingredient) (ΔΔCt- Value). The value obtained in this way is used in the equation n-fold expression (group A to group B) = 2 −ΔΔCt .
New quantification algorithms
In order to improve the accuracy of the relative quantification, various approaches, partly based on the ΔΔCt method, have been developed.
- Extension of the formula of the ΔΔCt method to include the efficiency of the respective PCR approach, which must be determined beforehand in a test run using a standard curve.
- By means of regression analyzes, fit the qPCR data sets to an exponential function or, in linearized form, to a straight line equation. The intersection of these functions with the y-axis then provides information about the amount of DNA originally present in the batch (relative information compared to a reference sample).
- Fit the qPCR data to a three- or multi-parameter sigmoid function. Here, too, the relative amount of DNA is determined via y (0).
The advantages of these new methods, most of which are not yet ready for the market, lie in the more precise analysis and lower variance of the PCR results. With some methods, the efficiency is taken into account in each individual sample preparation and thus enables a single tube analysis.
Reproducibility and comparability of the results
An experiment is worthless if it cannot be repeated. For this it is absolutely necessary to keep the test conditions constant. Not only must the consistency of the assay quality (primer, probes, polymerase, buffer, etc.) be taken into account, the devices must also meet the requirements. For example, in block systems (96 or 384 wells), so-called homogeneity is the major criterion. The assays must run equally well in every well of the block and also on blocks of identical devices. Furthermore, this homogeneity must not change over time. To ensure that the device can be used for safe experiments in the laboratory, appropriate controls should be carried out at regular intervals (some models are extremely prone to aging). An assay is analyzed in many replicates distributed over the entire block. Ideally, the result should be the same everywhere. Now the experimenter should be aware of the extent to which deviations can also have relevant effects. These deviations cannot be compensated for by means of expensive repetitions, since these would also be repeated constantly.
literature
- Bianca Holzapfel & Lucia Wickert (2007): The quantitative real-time PCR (qRT-PCR). In: Biology in Our Time. Volume 37, No. 2, pp. 120-126. doi : 10.1002 / biuz.200610332
- Michael Walter Pfaffl (2004): Real-time RT-PCR: New approaches to exact mRNA quantification. In: Biospectrum. PDF
- Bustin SA et al. (2009): "The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments." Clin Chem. 2009 Apr; 55 (4): 611-22. PDF
Web links
- www.gene-quantification.info English page with all knowledge, tips and tricks about qPCR
- realtimepcr.dk Real time PCR (Copenhagen)
- RefGenes Open Access online tool for the identification of tissue specific reference genes
- RT-PCR-article.pdf (PDF, 276 kB, good review article in English)
Individual evidence
- ↑ J. Nurmi, T. Wikman, M. Karp, T. Lövgren: high-performance real-time quantitative RT-PCR using lanthanide probes and a dual-temperature hybridization assay. In: Analytical chemistry. Volume 74, Number 14, July 2002, pp. 3525-3532, ISSN 0003-2700 . PMID 12139064 .
- ↑ A. Lehmusvuori, AH Tapio, P. Mäki-Teeri, K. Rantakokko-Jalava, Q. Wang, H. Takalo, T. Soukka: Homogeneous duplex polymerase chain reaction assay using switchable lanthanide fluorescence probes. In: Analytical biochemistry. Volume 436, Number 1, May 2013, pp. 16-21, ISSN 1096-0309 . doi : 10.1016 / year from 2013.01.007 . PMID 23353013 .

