Electrochemical cell
“ Electrochemical cell ” is a generic term for various arrangements that are either used in electrochemistry or that are based on electrochemical processes.
There are several main types of electrochemical cells, which differ in terms of whether they emit or absorb energy during operation: cells that generate an electrical voltage and can supply an electrical current are galvanic cells . Cells that are operated by an external current are electrolysis cells. Accumulators are cells that alternately deliver electricity and are then charged again by an externally applied current.
- Galvanic cells are electrochemical power sources that deliver usable electrical energy through chemical reactions at the chemically different electrodes.
- Electrolysis cells are used to obtain various substances by applying a voltage, see electrolysis .
- Like the galvanic cell, an accumulator cell serves as a power source; it is recharged by supplying energy. The charging process corresponds to electrolysis.
- Research or analysis cells for electroanalysis and electrochemistry can have three or more electrodes ( working electrode , reference electrode , counter electrode , possibly also indicator electrode (s)). If changing operating modes are possible, such cells cannot be clearly classified as electrolysis cells or as galvanic cells.
An electrochemical cell contains at least two electrodes , which always function as electron conductors, and at least one electrolyte , i. H. an ion conductor . The electrolyte can be liquid or solid, or both liquid and solid electrolytes are present. An electrochemical cell can thus be defined as an arrangement of two electrodes that are conductively connected via an electrolyte.
Examples of galvanic cells
A primary cell, usually colloquially called a “ battery ”, is an electrochemical cell, as is every fuel cell . Button cells are particularly small cells. Such commercially available electrochemical cells have a closed housing in order to prevent the escape of the electrolyte liquids. They therefore come closer to the ideal of a “cell” in the sense of an enclosed unit than some of the electrochemical cells used in research, which can also be open.
Examples of electrolytic cells
Schematic drawing of a Downs cell for the electrolysis of sodium chloride
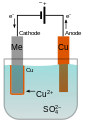
Scheme of an electrochemical cell for the deposition of copper
There are electrolyses that are used to produce basic chemicals such as B. the electrolysis of molten sodium chloride , which is used to represent the elements sodium and chlorine . The Downs cell was developed for this large-scale process . Then there is the electrolysis of electroplating , which coat objects with metal coatings. For this purpose, metal salt solutions such as the copper salt bath with copper sulfate CuSO 4 shown schematically in the figure are used . The Hull cell is used to test and optimize the deposition conditions in electroplating. The electrodes of the Hull cell are not parallel to each other. This results in higher current densities on the side where the electrodes are closer together. The influence of different current densities can be observed on an electrode, so that the best electrolysis conditions can be determined. The Haring-Blum cell is also a test cell in electroplating, with the help of two identical electrodes at different distances to examine the macroscopic ability .
Examples of cells for basic research
In basic electrochemical research, a three-electrode measuring arrangement is mostly used, as it allows the potential of an electrode, the working electrode , to be set independently of the state of the counter electrode . To operate a three-electrode cell, you need a suitable potentiostat or galvanostat and a reference electrode : These devices measure the voltage between the working and reference electrodes, with currents flowing between the working and counter electrodes.
A special type of research cell uses a rotating electrode, typically a rotating disk electrode ( RDE) at the end of a rod . This is rotated around the rod axis with the help of an electric motor, the speed of rotation being variable and known. A known flow profile is established in the liquid electrolyte, and the influence of the transport in the electrolyte on the electrode reaction can be studied. A variant of this is the rotating ring disk electrode ( RRDE), in which a further, ring-shaped electrode is attached around the disk electrode, likewise concentric to the axis of rotation.
Example of electrochemical sensors
A Clark cell is an electrochemical sensor for determining the oxygen concentration in a solution or in gases. In the diagram of such a Clark electrode shown, (A) is the cathode at which the oxygen is reduced. It is often made of platinum . (B) is an Ag / AgCl anode (C) is the KCl electrolyte and (D) is a Teflon membrane, which is permeable to the oxygen to be measured, but which keeps other disturbing environmental influences away from the measuring cell. (E) is a rubber ring and (F) is a voltage source used to operate the cell. The current measured with the sensitive current measuring device (G) is proportional to the oxygen concentration.
The Nernst probe is also an electrochemical cell as an oxygen sensor . It can be used as a lambda probe to control the engine of internal combustion engines , whereby it determines the residual oxygen content in the exhaust gas .
Basic properties
Important properties of an electrochemical cell are its instantaneous voltage ( operating voltage ) and, in the case of technical cells, its nominal voltage , as well as the open-circuit voltage that the cell assumes when no current is flowing. Due to the internal resistance of the cell and the resulting voltage drop , the operating voltage of a galvanic cell (battery) is below the open-circuit voltage. In the case of an electrolytic cell, on the other hand, where the flow of current is forced from the outside, the operating voltage is always above the open-circuit voltage. The reversible cell voltage is the rest voltage in the equilibrium state .