Potentiostat
A potentiostat is an electrical measuring device used in electrochemistry . In principle, it represents a special design of a control amplifier , which has three electrodes for the measurement : a working electrode , a high-resistance reference electrode and counter electrode, all three of which are connected to a galvanic element to be examined during operation . The potentiostat keeps an electrical voltage between the working electrode and the reference electrode constant via an electrical current between the counter electrode and the working electrode. The potentiostat measures the electrical voltage and the electrical current and outputs them as measured values.
The main applications for potentiostats are electrochemical studies. This includes, among other things, studies of chemical reactions that are caused by the electrical current, such as in electrolysis, or that are caused by an electrical current, such as in batteries or fuel cells . They are also used to characterize electrodes and electrolytes , i.e. ion-conducting liquids or solids, for example in analysis . In addition to measurements that are constant over time, potentiostats can usually also execute various signal curves such as voltage ramps or be coupled with external signal generators and thus used, for example, for cyclic voltammetry .
The first work on the construction of a potentiostat as a measuring device in electrochemistry goes back to A. Hickling in 1941. A measuring device with a related function is the galvanostat , in which an electric current is regulated and kept constant by setting an electric voltage on the counter electrode.
Mode of action
In the case of a potentiostat, the electrode potential , i. H. the voltage of an electrode with respect to a reference point is regulated to a desired value. For this purpose, the electric current between the working electrode and the counter electrode, which is also called the auxiliary electrode, is adjusted by the potentiostat so that the desired potential is reached. A third electrode, the reference electrode and whose potential is defined in the electrochemical series , represents the reference point to the working electrode. So that the reference electrode maintains its potential unchanged, it is necessary that no current flows through it itself; this is guaranteed by a very high-resistance input on the potentiostat.
Another special feature compared to other control amplifiers is that, on the one hand, a very high gain is required to keep the control deviation low: Accuracies of 1 mV or less are required when measuring the voltage at the reference electrode. On the other hand, the regulation should take place very quickly, typical regulation time constants are 10 µs or less. The controlled system between the working electrode and the counter electrode thus represents an impedance with an ohmic resistance and a capacitive component that can change rapidly over time. Potentiostats must be able to regulate such unknown loads without becoming unstable, i.e. H. to start vibrating .
Potentiostats can also be used as voltage measuring devices and have a significantly higher internal resistance compared to digital multimeters . The nominal input impedance of a digital multimeter is typically 10 MΩ, that of a typical potentiostat is 10 GΩ. Thanks to the control amplifier, the potentiostat can also be used as an ammeter with an internal resistance of 0 Ω, since the current is actively controlled.
Practical execution
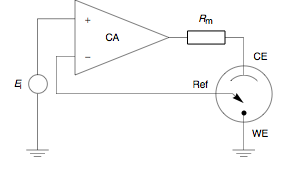
In principle, potentiostats are implemented with an operational amplifier , as shown in the figure opposite, in whose non-inverting input the desired potential is fed as an electrical voltage with respect to ground. The potential of the reference electrode is fed into the inverting input. The working electrode is then connected to ground, the counter electrode to the output of the operational amplifier. The operational amplifier regulates the output current in such a way that the inverting input is always at the same voltage level as the non-inverting input, so that the desired potential is adjusted. The resulting current at the output of the operational amplifier can then be measured and displayed next to the reference potential on a display or made available for further data processing by means of telemetry .
Regulator control
The operational amplifier can be replaced by a regulator which stabilizes the voltage between the reference electrode and the working electrode. This algorithm is based on a ratio equation :
- . (1)
- is the last measured voltage between the working electrode and the counter electrode, i.e. the cell voltage .
- is the last measured electrochemical potential , i.e. the voltage between the reference electrode and the working electrode that is to be stabilized.
- is the calculated next cell voltage of the controller, i.e. the result of the changed equation.
- is the setpoint , i.e. the desired potential .
Since equation 1 is basically a P controller , the measurement interval should remain constant. The algorithm then calculates that it is as close as possible to the target value . The algorithm requires the use of the following computer-controlled devices: digital voltmeter , power supply and a relay to switch the polarity of the cell voltage if necessary.
Embodiments
Potentiostats are commercially available in a variety of configurations. Most devices can now be controlled with a PC , some potentiostats are even used as plug-in cards in the measuring computer. Many potentiostats have analog outputs that allow current and voltage to be recorded with a recorder or storage oscilloscope. The main differentiating features between the models are essentially the available current and voltage ranges. Typical currents in non-industrial applications are in the range from a few nA to a few A. The potential control is usually in the range of ± 10 V, a few devices can regulate potentials up to around 50 V. The counter electrode voltage is between ± 10 and ± 50 V for standard devices; devices with control voltages above 100 V are offered for poorly conductive electrolytes.
There are technical differences both in the actual potentiostatic control circuit and in the type of current measurement. So z. B. either the working electrode or the reference electrode can be related to ground. If the working electrode is grounded, there are advantages through simple construction and high stability against vibrations. If the reference electrode is referenced to ground, several working electrodes can be operated independently of one another in a common container ( bi-potentiostat ).
In the simplest case, the current is measured in the counter electrode circuit. If the current is measured across a resistor in the working electrode circuit, another assembly (differential generator) must correct the potential error caused by this current measurement. This type of current measurement is preferably used for devices that are supposed to deliver high currents. A variant of the current measurement consists in using a zero ohm ammeter in the working electrode circuit. Although this variant is technically complex, it can measure currents down to the pA range and below.
Web links
- Application examples and areas of application for potentiostats
- Here you will find information on the use of potentiostats as potentiostat, galvanostat, power source, zero ohm ammeter and the like. a. m.
- Potentiostat basics
- Explanations of specifications of potentiostats /
Individual evidence
- ↑ Rudolf Dölling: Potentiostats - An Introduction. 2004, accessed March 27, 2018 .
- ^ A. Hickling: Studies in electrode polarization. Part IV.-The automatic control of the potential of a working electrode . In: Transactions of the Faraday Society . 38, 1942, pp. 27-33. doi : 10.1039 / TF9423800027 .
- ↑ Potentiostat stability mystery explained ( Memento of the original from October 23, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF file; 127 kB).
- ^ M. Siegert: A scalable multi-channel software potentiostat . In: Frontiers in Energy Research . 6, 2018, p. 131. doi : 10.3389 / fenrg.2018.00131 .