Ethyl lauryl arginate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Ethyl lauryl arginate | ||||||||||||
| other names | |||||||||||||
| Molecular formula |
|
||||||||||||
| Brief description |
white powder (hydrochloride) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
50.5–58 ° C (hydrochloride) |
||||||||||||
| solubility |
readily soluble in water, ethanol, propane-1,2-diol and glycerol (hydrochloride) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Ethyl lauryl arginate (short: LAE ) is an organic-chemical compound that is derived from lauric acid. Hydrochloride is used almost exclusively , which is approved as a preservative with the number E 243 in processed meat products and as a cosmetic additive for many cosmetics in the EU.
Extraction and presentation
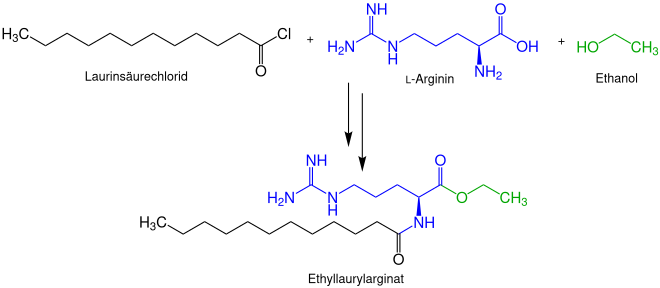
Ethyl lauryl arginate can be produced in two steps from the acid chloride of lauric acid , L-arginine and ethanol .
In the first step, the carboxy group of the amino acid arginine is esterified with ethanol, whereby ethyl arginate is formed. For this purpose, thionyl chloride as a catalyst.
In the second step, the ethyl arginate is reacted with lauric acid chloride to form ethyl lauryl arginate. The α-amino group of the ethyl arginate is converted to the amide. The product is obtained as the hydrochloride. Since only the naturally occurring and cheaper L -arginine is used, only the ( S ) -stereoisomer is formed.
properties
Ethyl lauryl arginate hydrochloride is effective against a large number of gram-positive and gram-negative bacteria, yeasts and molds. It prevents the growth of microorganisms by acting on the cell membrane and cytoplasm. However, there is no complete cell lysis .
Usage and Limitation
Due to its properties, the hydrochloride of ethyl lauryl arginate is mainly used as a preservative in food and cosmetics.
In the food sector, it may only be used as such in the EU for heat-treated, processed meat in order to improve the microbiological quality and to prevent the growth of microorganisms.
The permitted additional amount is 160 mg / kg and the permitted daily dose is set by the EFSA at 0.5 mg per kilogram and day. In the USA the substance has the status Generally Recognized As Safe (GRAS).
In Germany it can be used in many cosmetics. Soaps, anti-dandruff shampoos and roll-ons may contain up to 0.8%, and up to 0.4% in other cosmetics apart from lip products, oral products and sprays. Ethyl lauryl arginate can also be found in natural cosmetics, as it can be made from natural raw materials.
It is also being investigated as a possible antimicrobial additive in food packaging.
Individual evidence
- ↑ Entry on E 243: Ethyl lauroyl arginate in the European database for food additives, accessed on June 28, 2020.
- ↑ a b c d e f g Entry on ethyl lauryl arginate. In: Römpp Online . Georg Thieme Verlag, accessed on December 4, 2019.
- ↑ External identifiers or database links to Ethyllauroylarginate Hydrochloride : CAS number: 60372-77-2, EC number: 434-630-6, ECHA InfoCard: 100.103.285 , GESTIS substance database : 536097 , PubChem : 25229630 , ChemSpider : 32701644 , Wikidata : Q27293951 .
- ↑ a b c data sheet Ethyl Lauroyl Arginate from Sigma-Aldrich , accessed on December 4, 2019 ( PDF ).
- ^ A b c Yoko Kawamura & Brian Whitehouse: Ethyl Lauroyl Arginate - Chemical and Technical Assessment . 2008 ( fao.org [PDF; accessed December 5, 2019]).
- ↑ Patent EU1294678 : Process for the production of cationic surfactants. Published on August 3, 2006 , inventors: Agustin Contijoch Mestres, Francisco Javier, Rodriguez Martinez & Joan Seguer Bonaventura.
- ↑ E 243 ethyl lauryl arginate. Retrieved December 5, 2019 .
- ↑ Federal Ministry of Food, Agriculture and Consumer Protection: Fifty-fifth ordinance amending the Cosmetics Ordinance . Ed .: Federal Law Gazette 2010 Part I No. 42. August 9, 2010 ( bgbl.de [PDF; accessed December 5, 2019]).
- ^ V. Muriel-Galet, GL Carballo, P. Hernández-Muñoz & R. Gavara: Ethyl Lauroyl Arginate (LAE): Usage and Potential in Antimicrobial Packaging . In: Jorge Barros-Velázquez (Ed.): Antimicrobial Food Packaging . Academic Press, 2016, ISBN 978-0-12-800723-5 , chap. 24 , p. 313-318 , doi : 10.1016 / B978-0-12-800723-5.00024-3 .