Gallium (I, III) chloride
| Crystal structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
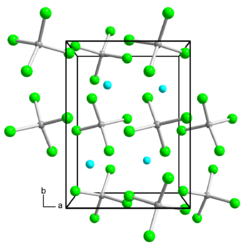
|
|||||||||||||
| __ Ga + __ Ga 3+ __ Cl - | |||||||||||||
| Crystal system |
tetragonal |
||||||||||||
| Lattice parameters |
a = 7.2235 Å , b = 9.7211 Å, c = 9.5421 Å |
||||||||||||
| General | |||||||||||||
| Surname | Gallium (I, III) chloride | ||||||||||||
| other names |
|
||||||||||||
| Ratio formula | GaCl 2 | ||||||||||||
| Brief description |
colorless solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 140.62 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
2.42 g cm −3 |
||||||||||||
| Melting point |
172.4 ° C |
||||||||||||
| boiling point |
535 ° C (beginning of decomposition above 200 ° C) |
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Gallium (I, III) chloride is an inorganic chemical compound of gallium from the group of chlorides .
Extraction and presentation
Gallium (I, III) chloride can be obtained by reacting gallium with gallium (III) chloride at 180 ° C in the absence of moisture.
It is also possible to display it by reacting gallium with hot hydrochloric acid .
properties
Gallium (I, III) chloride is a colorless, hygroscopic solid that is soluble in benzene and is used as a strong reducing agent. It is in crystalline form and in the melt as Ga [GaCl 4 ]. In molten form, the compound conducts electricity and does not wet glass. Slow cooling of the clean melt leads to a supercooled melt , which can be liquid for years even at room temperature. The lattice constants of the tetragonal unit cell are a = 7.2235 Å , b = 9.7211 Å, c = 9.5421 Å.
Individual evidence
- ↑ a b c d e f g Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 853.
- ^ Egon Wiberg, Nils Wiberg: Inorganic Chemistry . Academic Press, 2001, ISBN 0-12-352651-5 , pp. 1036 ( limited preview in Google Book search).
- ↑ a b c d e Laurence S. Foster: Gallium (II) chloride . In: JC Bailar, Jr. (Ed.): Inorganic Syntheses . tape 4 . McGraw-Hill, Inc., 1953, pp. 111-114 (English).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ S. Chand: Advanced Inorganic Chemistry . 2000, ISBN 81-219-0263-0 , pp. 801 ( limited preview in Google Book search).
- ↑ G. Singh: Chemistry Of D-Block Elements . Discovery Publishing House, 2007, ISBN 81-8356-242-6 , pp. 61 ( limited preview in Google Book search).
- ^ AP Wilkinson; AK Cheetham: Study of oxidation ‐ state contrast in gallium dichloride by synchrotron X ‐ ray anomalous scattering . In: Acta Crystallographica Section B . tape 47 , no. 2 , April 1991, pp. 155–161 , doi : 10.1107 / S0108768190010485 (English).

