Gold (III) fluoride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Au 3+ __ F - | ||||||||||
| Crystal system |
hexagonal |
|||||||||
| Space group |
P 6 1 22 (No. 178) |
|||||||||
| Lattice parameters |
a = 515.08 pm |
|||||||||
| General | ||||||||||
| Surname | Gold (III) fluoride | |||||||||
| other names |
Gold trifluoride |
|||||||||
| Ratio formula | OnF 3 | |||||||||
| Brief description |
orange-yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 253.96 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
6.75 g cm −3 |
|||||||||
| Melting point |
300 ° C (sublimation) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Gold (III) fluoride is an inorganic chemical compound of gold from the group of fluorides .
Extraction and presentation
Gold (III) fluoride can be obtained by pyrolysis of gold (V) fluoride at 200 ° C or by fluorination of gold (III) chloride .
It can also be made by fluorinating gold with bromine (III) fluoride .
properties
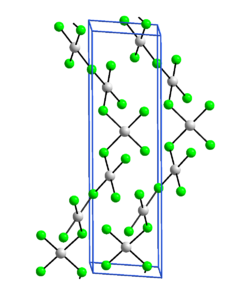
Gold (III) fluoride is a diamagnetic orange-yellow solid. It decomposes at 500 ° C. Its crystal structure is isotypic to that of silver (III) fluoride ( hexagonal , space group P 6 1 22 (space group no. 178) , and is composed of square-planar AuF 4 units, each AuF 4 unit being with two others Units connected to form spiral chains.
With fluoride ions, gold (III) fluoride forms fluoroaurates (III) [AuF 4 ] - and [Au 2 F 7 ] - . The [AuF 4 ] - ion also occurs in Au 3 F 8 (gold (II) bis-tetrafluoroaurate (III)).
use
Gold (III) fluoride can be used to make gold azides .
Individual evidence
- Jump up ↑ B. Zemva, K. Lutar, A. Jesih, WJ Casteel Jr., AP Wilkinson, DE Cox, RB von Dreele, H. Borrman, N. Bartlett: Silver Trifluoride: preparation, crystal structure, some properties, and comparison with OnF 3 . In: Journal of the American Chemical Society , 1991 , 113 , pp. 4192-4198 doi : 10.1021 / ja00011a021 .
- ↑ a b c d e Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . Taylor & Francis US, 2011, ISBN 1-4398-1461-9 , pp. 191 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b Erwin Riedel, Christoph Janiak: Inorganische Chemie . Walter de Gruyter, 2011, ISBN 3-11-022567-0 , p. 759 f . ( limited preview in Google Book search).
- ↑ Mido & S. Taguchi: Chemistry in Aqueous and Non-aqueous Solvents . Discovery Publishing House, 1997, ISBN 81-7141-331-5 , pp. 158 ( limited preview in Google Book search).
- ^ R. Schmidt, B. G. Müller: Single crystal investigations on Au [AuF 4 ] 2 and CeF 4 , two unexpected by-products . In: Journal for inorganic and general chemistry , 1999 , 625 , pp. 605-608 ( doi : 10.1002 / (SICI) 1521-3749 (199904) 625: 4 <605 :: AID-ZAAC605> 3.0.CO; 2-6 ).
- ↑ Herbert W. Roesky: Efficient Preparations of Fluorine Compounds . John Wiley & Sons, 2012, ISBN 1-118-40942-6 , pp. 96 ( limited preview in Google Book search).