Gold (III) selenate
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
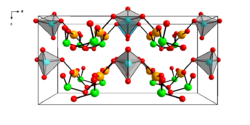
|
||||||||||
| __ Au 3+ __ Se 4+ __ Se 6+ __ O 2− | ||||||||||
| General | ||||||||||
| Surname | Gold (III) selenate | |||||||||
| other names |
Gold triselenate |
|||||||||
| Ratio formula | Au 2 (SeO 4 ) 3 | |||||||||
| Brief description |
yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 822.81 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
370 ° C (decomposition) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Gold (III) selenate is the historical name of the reaction product of gold with selenic acid .
According to more recent sources, however, it can be assumed that the so-called chemical compound does not belong to the group of selenates , but that it is a selenite selenate .
Extraction and presentation
Gold (III) selenate can be obtained by reacting hot concentrated selenic acid with gold. More recent sources give the composition of the resulting compound as Au 2 (SeO 3 ) 2 (SeO 4 ), which is why it should be referred to as gold selenite selenate.
The dissolution of elemental gold with concentrated selenic acid to form gold selenates was described for the first time by Eilhard Mitscherlich in 1827 . V. Lenher was able to further prove the existence of gold selenates by isolating yellow crystals, to which he assigned the molecular formula Au 2 (SeO 4 ) 3 . In X-ray structure examinations of the compounds shown and structurally characterized, they all turned out to be selenites or diselenites of gold.
properties
Gold (III) selenate is a yellow solid that is practically insoluble in water. However, it turns dark under light and air. The compound has an orthorhombic crystal structure with the space group Cmc 2 1 (space group no. 36) with the lattice parameters a = 16.89 Å , b = 6.301 Å and c = 8.327 Å. The unit cell contains four formula units. The Au 3+ ion is surrounded by four oxygen atoms in a square planar manner . The compound reacts with perchloric acid to form the triclinic gold (III) selenite chloride Au (SeO 3 ) Cl.
Individual evidence
- ↑ a b c d e William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 102 ( limited preview in Google Book search).
- ^ A b c Mathias S. Wickleder, Oliver Büchner, Claudia Wickleder, Sherif el Sheik, Gunther Brunklaus, Hellmut Eckert: Au2 (SeO3) 2 (SeO4): Synthesis and Characterization of a New Noncentrosymmetric Selenite − Selenate. In: Inorganic Chemistry. 43, 2004, p. 5860, doi : 10.1021 / ic049270z .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Hermann Sicius: chalcogens: Elements of the sixth main group A journey through the periodic table . Springer-Verlag, 2015, ISBN 978-3-658-10522-8 , pp. 28 ( limited preview in Google Book search).
- ↑ a b Oliver Büchner: Coin metals with and in complex anions: synthesis, structure and properties , dissertation, Faculty of Mathematics and Natural Sciences at the Carl von Ossietzky University of Oldenburg, 2005.
- ↑ E. Mitscherlich, Pogg. Ann. 1827, 9, 623.
- ↑ V. Lenher, J. Am. Chem. Soc. 1902, 24, 354.
- ↑ PG Jones, E. Schwarzmann, GM Sheldrick, H. Timpe: Representation and structure of di-gold (III) to (selenite) (diselenite), Au 2 (SeO 3 ) 2 (Se 2 O 5 ). In: Journal of Nature Research B . 36, 1981, pp. 1050-1051 ( online ).
- ↑ PG Jones, GM Sheldrick, E. Schwarzmann, A. Vielmäder: Preparation and Crystal Structure of di-gold (III) to (selenite) oxide, Au 2 (SeO 3 ) 2 O. In: Zeitschrift für Naturforschung B . 38, 1983, pp. 10-11 ( PDF , free full text).
- ^ John Raymond Mickelsen: Coloring Glass with Gold Selenate . Oregon State College, 1953.
- ↑ Peter G. Jones, Martin Kraushaar, Einhard Schwarzmann, George M. Sheldrick: Representation and crystal structure of gold (III) selenite chloride, Au (SeO 3 ) Cl. In: Journal of Nature Research B . 37, 1982, pp. 941-943 ( PDF , free full text). doi : 10.1515 / znb-1982-0802 .
