Hexachloropropene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
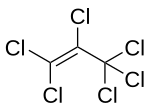
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Hexachloropropene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 Cl 6 | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 248.75 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.76 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
-73 ° C |
||||||||||||||||||
| boiling point |
209–210 ° C (1013 hPa) |
||||||||||||||||||
| Vapor pressure |
30 hPa (100 ° C) mbar |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.548 (20 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Hexachloropropene is a chemical compound from the group of chlorinated hydrocarbons .
Extraction and presentation
Hexachloropropene can be obtained by dehydrochlorination of 1,1,1,2,2,3,3-heptachloropropane with alkali metal hydroxides such as potassium hydroxide in alcoholic solution.
properties
Hexachloropropene is a colorless liquid that is sparingly soluble in water.
use
Hexachloropropene is used to produce other chemical compounds such as B. uranium (IV) chloride or anhydrous niobium (V) chloride and tungsten (VI) chloride are used.
Individual evidence
- ↑ a b c d e f g h i j k data sheet hexachloropropene (PDF) from Merck , accessed on April 21, 2013.
- ^ SD Gangolli, Royal Society of Chemistry (Great Britain): The Dictionary of Substances and Their Effects: D. Vol . 3 . Royal Society of Chemistry, 1999, ISBN 0-85404-818-9 , pp. 607 ( limited preview in Google Book search).
- ↑ Patent US5763705 : Method of producing 1,1,1,3,3-pentafluoropropane, a method of producing 1,1,1,3,3-pentafluoro-2-halogeno-3-chloropropane, and a method of producing 1, 1,1,2,3,3-hexachloropropene. Filed on Oct. 3, 1997 , Applicant: Daikin Industries Ltd ..
- ^ WW Porterfield and SY Tyree, Jr .: Anhydrous metal chlorides . In: S. Young Tyree, Jr. (Ed.): Inorganic Syntheses . tape 9 . McGraw-Hill Book Company, Inc., 1967, p. 133-136 (English).
