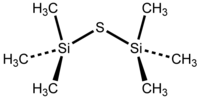
Hexamethyldisilathiane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexamethyldisilathiane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | (CH 3 ) 3 SiSSi (CH 3 ) 3 | |||||||||||||||
| Brief description |
colorless liquid with a strong odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 178.44 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.846 g cm −3 (25 ° C) |
|||||||||||||||
| boiling point |
164 ° C |
|||||||||||||||
| Refractive index |
1.4586 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Hexamethyldisilathiane is a chemical compound from the group of trimethylsilyl compounds .
Extraction and presentation
Hexamethyldisilathiane can be obtained by reacting trimethylsilyl chloride with hydrogen sulfide and pyridine .
properties
Hexamethyldisilathiane is a colorless liquid with a strong odor.
use
Hexamethyldisilathiane is mainly used to convert oxides and chlorides into the corresponding sulfides.
Individual evidence
- ↑ a b c d e f g h Data sheet Hexamethyldisilathian, synthesis grade from Sigma-Aldrich , accessed on January 15, 2014 ( PDF ).
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 713.
- ^ Sonny C. Lee, Richard H. Holm: Nonmolecular Metal Chalcogenide / Halide Solids and Their Molecular Cluster Analogues. In: Angewandte Chemie International Edition in English. 29, 1990, pp. 840-856, doi : 10.1002 / anie.199008401 .


![{\ displaystyle \ mathrm {2 \ (CH_ {3}) _ {3} SiCl + H_ {2} S + 2 \ C_ {5} H_ {5} N \ longrightarrow [(CH_ {3}) _ {3} Si] _ {2} S + 2 \ C_ {5} H_ {5} N \ cdot HCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0415a858f729982131dba59b4d4dc4ccffbd76df)