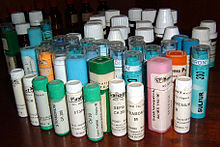
Homeopathic medicine
Homeopathic medicinal product is the name of a medicinal product that has been manufactured using a homeopathic preparation process. The terms homeopathic ( plural : homeopathic ) or homeopathic preparation are used synonymously .
A homeopathic drug can contain one or more active ingredients within the meaning of the homeopathic active ingredient term. However, the homeopathic concept of active ingredient does not correspond to the chemical term or the medical effectiveness : Homeopathic medicines do not have to contain a molecule of the active ingredient. For homeopathic medicines there is no scientific evidence that the effectiveness goes beyond that of placebos (dummy drugs). See also criticism of homeopathy . The largest markets for homeopathic medicines are in France, USA, Germany and India.
Single remedies and complex remedies
Homeopathic medicines differ according to their basic substances, their dilutions ( potencies ) and their forms of administration ( dilutions , tablets , globules , triturations, ampoules , ointments, etc.). Sometimes there are also unpowered homeopathic medicines, such as the mother tincture . For the ingredients see “ List of basic homeopathic substances ”.
Complex remedies or combination preparations are homeopathic remedies that are a mixture of two or more individual homeopathic remedies with different or the same potentiation or dilution. In Germany, just over half of the homeopathic finished medicinal products are such complex products . Complex remedies, in contrast to the individual homeopathic remedies , have not been examined on healthy people by means of a drug test according to the rules of classical homeopathy. In complex therapy, the individual remedies should complement and reinforce each other's effects. Low potencies are mostly used for this. Complex remedies often have a therapeutic notice and are subject to approval. The term complex remedy is still used outside of homeopathy for Bach flower remedies and the so-called " Schuessler salts ".
Active ingredient term
For homeopathic medicinal products, the term “active ingredient” differs from that which is commonly used in pharmacology and pharmacy . While there the medicinal substance (drug) is usually the starting material in terms of quantity to which the effect is attributed, the European Pharmacopoeia extends the term of the active ingredient for a homeopathic medicinal product to include the dilution or trituration of the starting material or starting material . The starting material can be a concentrated preparation of vegetable, animal or human origin (such as the mother tincture) or a chemical or mineral substance.
For example, the indication of the active ingredient content for a homeopathic medicinal product Calcium sulfuricum D6 tablets could be: "One tablet contains 250 mg Calcium sulfuricum Trituratio D6." Even if it were mathematically correct, it is not permissible according to the pharmacopoeia definition to refer to the starting substance take and express the active ingredient content as: "One tablet contains 0.25 µg Calcium sulfuricum."
Highly potentised preparations contain practically no molecule of the starting substance due to the high degree of dilution.
- → Main article: Potentiation (homeopathy)
Legal classification
European Union
With Directive 2001/83 / EC , an essential part of pharmaceutical law in the European Union was harmonized. This also affects homeopathic medicines. Article 1 of the directive contains the following definition:
- “Any medicinal product that has been prepared from substances called homeopathic stocks in accordance with a homeopathic preparation process described in the European Pharmacopoeia or, in the absence thereof, in the currently officially used pharmacopoeia of the Member States. A homeopathic medicine can also contain several active ingredients. "
Germany and Austria have only slightly modified this wording in their national pharmaceutical laws.
The Homeopathic Pharmacopoeia (HAB) and the Pharmacopée Française (Ph.F.) contain descriptions of homeopathic preparation methods that go beyond those described in the European, German or Austrian pharmacopoeia.
Registration
With Directive 2001/83 / EC, a simplified procedure for the approval of certain homeopathic medicinal products was established across the EU. Article 13 (2) stipulates: “The Member States shall create a particularly simplified registration procedure for homeopathic medicinal products within the meaning of Article 14.” The explanatory memorandum of the EU directive states: “In view of the peculiarities of homeopathic medicinal products, such as their very low active ingredient concentration, and the difficulty of using the conventional statistical methods in clinical trials, it appears desirable to provide a particularly simplified registration procedure for those homeopathic medicinal products that are used without therapeutic indication and in a preparation and a dosage that pose no risk to the patient Be brought into circulation. ” (Greetings No. 21). The simplified procedure can be used for homeopathic medicinal products if they lack a special indication of healing, they are intended for external or oral use, the degree of dilution is at least 1: 10,000 (corresponding to a potency of D4 / C2) and certain concentrations require a prescription Substances are not exceeded. No proof of efficacy has to be provided, it is only checked whether the medicinal products are manufactured according to the homeopathic preparation methods described in the European Pharmacopoeia or in another pharmacopoeia of a member state and whether the manufacturer can prove their quality and safety. The transposition into national law was mandatory for all member states.
In Germany, such finished medicinal products are entered in a register for homeopathic medicinal products. Special labeling requirements apply to them, for example that no therapeutic indication may be given. The information reads: "Registered homeopathic medicinal product, therefore no indication of a therapeutic indication" .
The majority of the homeopathic medicinal products on the market in Germany are registered products.
Admission
For homeopathic medicinal products that do not meet the requirements for a simplified procedure such as registration (for example, if they are to carry a medical notice), authorization must be applied for , as is the case for other medicinal products .
For these homeopathic medicinal products that are subject to authorization, an EU member state can introduce or maintain special regulations for the preclinical and clinical trials of these medicinal products in accordance with its own principles and special characteristics of homeopathic medicine. 12 countries (including Germany and Austria) list corresponding special regulations in their national pharmaceutical laws. 6 countries have no regulations deviating from the EU approval regulations for homeopathic medicinal products and 10 countries allow the establishment of special national regulations, but have not published them or do not apply them in practice.
Germany
Approved homeopathic medicinal products have an indication that is usually introduced in the package insert with the words: “The areas of application are derived from the homeopathic medicinal product pictures. These include: [...] ". The area of application must be verified by the manufacturer in the approval procedure at the Federal Institute for Drugs and Medical Devices (BfArM) with clinical studies or other "scientific knowledge material". While for conventional drugs it is necessary to check the tolerability and proof of effectiveness through clinical studies according to scientific standards for approval, for homeopathic drugs according to the principle of internal consensus, for example, only homeopathic literature, observational studies or monographs drawn up by the Commission D responsible for homeopathics are sufficient . Commission D, with the participation of the BfArM, adopted a criteria scheme that describes the evaluation criteria for the evidence presented depending on the severity of the disease to be treated. Clinical evidence of effectiveness would therefore only be necessary for homeopathic drugs for the treatment of serious diseases, but such evidence has not yet been provided for a single homeopathic drug.
As of April 2016, there were 4945 marketable homeopathic products, 2782 of which were combination preparations (“complex products”). A quarter of homeopathic medicinal products have completed an approval or re-approval process. With very few exceptions, homeopathics are only sold in pharmacies in Germany.
Austria
In Austria, approved homeopathic medicinal products have indications, no restrictions on potency and can be brought onto the market in all medicinal forms. Instead of results from clinical trials, “documents on the specific homeopathic effectiveness” in the approval application are sufficient. Preclinical and clinical data are not required, a toxicological assessment and the justification for homeopathic use are required.
France
The applicant for authorization is exempt from submitting all or part of the pharmacological, toxicological and clinical results, taking into account the specifics of a homeopathic medicinal product, provided that he can demonstrate that the homeopathic use of the medicinal product is safe on the one hand and well established in France on the other.
Other member states
Other member states that also list special regulations for the approval of homeopathic medicinal products in their national medicinal product laws are Belgium, Bulgaria, Finland, Ireland, Latvia, Lithuania, Portugal, the Czech Republic and the United Kingdom. The permissible indications are largely limited to mild diseases.
Prescription requirement
Homeopathic medicinal products can have pharmacodynamic and toxic effects if they still contain the corresponding starting substances in a sufficiently high concentration (equivalent to low potency) . The potency D4 is drawn as the limit here according to the drug prescription regulation (Germany) or the prescription regulation (Austria). Therefore, some homeopathic medicines are subject to prescription requirements .
Switzerland
In the Therapeutic Products Act (HMG) of Switzerland, homeopathic medicinal products are defined as "which are exclusively based on the basic principles of the Pharmacopoeia, the German Homeopathic Pharmacopoeia (HAB), the Pharmacopée Française (Ph.F .; under préparations homéopathiques) or in the British Homeopathic Pharmacopoeia (B.Hom.P.) described homeopathic manufacturing processes contain homeopathic active ingredients and are intended for use according to the principles of a homeopathic therapy direction . " According to the Swiss HMG, homeopathic medicinal products include homeopathic single remedies, homeopathic potency accords, homeopathic complex remedies, homeopathic - spagyric and spagyric medicines, anthroposophic medicines, Schüßler salts , nosodes , as well as preparations of animal origin, organ preparations and other active ingredients that are manufactured according to homeopathic or anthroposophic manufacturing instructions. For them, as for certain other complementary and phytotherapy drugs, a simplified approval process is possible. Special regulations apply for the proof of therapeutic efficacy and safety.
United States
In the United States, finished homeopathic medicines can be marketed provided that they meet the requirements set by the Food and Drug Administration (FDA). The remedy must be listed in the Homeopathic Pharmacopeia of the United States ( HPUS ) and have specific packaging and labeling. Non-prescription homeopathic medicines must state (at least) one area of application on their label. The HPUS contains around 1,350 monographs with raw materials and their preparations. It also describes the manufacturing process for homeopathic dosage forms and defines their properties and quality. Proof of efficacy is not required for a drug to be included in HPUS.
Canada
In Canada, homeopathic medicines are classified as "Natural Health Products" and can only be marketed with approval. Pharmaceutical companies must submit an application to the Natural Health Products Directorate (NHPD), which must be accompanied by documents on quality, safety and effectiveness. The efficacy can optionally be demonstrated on one of five levels of evidence, ranging from clinical studies of various quality to bibliographic evidence to references to traditional use. Approved products have a registration number ("Drug Identification Number-Homeopathic Medicine", DIN-HM ).
market
In 2013, the share of homeopathic medicines in the German pharmacy market was 1.3% of sales, and 3.9% of the number of units sold. In a survey carried out by the Allensbach Institute in Germany in 2014 , 94% of the population had already heard of homeopathic medicines.
Worldwide sales of homeopathic medicines made up 0.3% of the world market for medicines in 2007 at 1.5 billion euros (manufacturer price). More than 70% of homeopathic medicines are sold in Western Europe (by value). France is the most important market in 2010 with 323 million euros, followed by Germany (302 million euros). In France, only doctors are allowed to prescribe homeopathic treatments. In 2012, around one in four general practitioners in France also used homeopathy. The French government reduced the reimbursement for prescription homeopathics to 30 percent, but Paris lifted the 1988 ban on price increases for prescription homeopathics.
In terms of packs sold, India is by far the largest market for homeopathic medicines.
Individual evidence
- ^ A b Hermann PT Ammon: Hunnius Pharmaceutical Dictionary . De Gruyter 2004, page 732. ISBN 3-11-017475-8 .
- ↑ Section 4 (26) AMG
- ^ Edzard Ernst : The truth about homeopathy. (PDF; 57 kB) Br J Clin Pharmacol . 2008 Feb; 65 (2): 163-4. Epub 2007 Sep 13. PMID 17875194
- ↑ HH. Frey and W. Löscher: Textbook of pharmacology and toxicology for veterinary medicine. Enke Verlag Stuttgart, ISBN 978-3-432-26941-2 , chapter "Homeopathy".
- ↑ Market significance and acceptance of homeopathic medicines , Federal Association of Drug Manufacturers (BAH), accessed in February 2014.
- ↑ Drug Information System (AMIS) of the German Institute for Medical Documentation and Information [1]
- ↑ a b c Statistics “Special Therapies and Traditional Medicines” ( Memento from April 30, 2017 in the Internet Archive ), BfArM, as of April 2016.
- ↑ Homeopathy - single and complex remedies [2]
- ↑ Monograph Homeopathic Preparations , 8th Supplement to the European Pharmacopoeia, 5th Edition
- ↑ Germany: Section 4 Paragraph 26 AMG , Austria: Section 1 Paragraph 10 AMG
- ↑ a b Directive 2001/83 / EC of the European Parliament and of the Council of 6 November 2001 on the creation of a Community code for medicinal products for human use, consolidated version (English)
- ↑ Implementation in the German Medicines Act in Section 38 AMG , Section 39 AMG , in the Austrian Medicines Act in Section 11 AMG
- ↑ § 10 AMG
- ↑ European Coalition on Homeopathic and Anthroposophic Medicinal Products (ECHAMP) - EU Policy and Legislation - National Implementation , accessed on July 15, 2019.
- ↑ § 22 Medicines Act (AMG).
- ↑ a b Katrin Schumacher: Alternative medicine. I. Alternative medicine: Limits of medical therapy freedom under medical liability, drug law and social law (Kölner Schriften zum Medizinrecht, Volume 20), Springer (2017). P. 137 ff
- ↑ Special legal regulation according to § 2 of the drug testing guidelines regulation (AMPV), in connection with § 26 Abs. 2 AMG.
- ↑ Criteria for knowledge material on clinical indications in homeopathy , BfArM, October 9, 2002.
- ↑ a b maiLab . (July 11, 2019). Homeopathy law: Germany's worst law . funk .
- ↑ Why this pharmacist no longer offers homeopathics . The daily mirror. August 22, 2018. Retrieved July 26, 2019.
- ↑ #Faktenfuchs: Dispute about homeopathy . Bavarian radio. June 8, 2019. Accessed July 26, 2019.
- ↑ Criticism of the approval of homeopathic medicinal products . Deutschlandfunk. June 14, 2019. Retrieved July 26, 2019.
- ↑ "From my point of view, this is pharmaceutical advertising" . Mirror online. December 6, 2016. Accessed July 26, 2019.
- ↑ § 9 b AMG
- ↑ a b c HMPWG Report on the Regulatory Status of Homeopathic Medicinal Produts for Human Use in EU and EFTA Countries - 2016 .
- ^ Ordinance on the prescription requirement for medicinal products (AMVV) § 5 [3]
- ↑ Ordinance of the Federal Minister for Health and Environment Protection on Prescription Drugs [4]
- ↑ a b Institute Council of the Swiss Therapeutic Products Institute: Ordinance of the Swiss Therapeutic Products Institute on the simplified authorization of complementary and herbal medicinal products 2005 (PDF)
- ^ Food and Drug Administration: Conditions Under Which Homeopathic Drugs May Be Marketed. Section 400.400 of the Compliance Policy Guides Manual (CPG)
- ^ Homeopathic Pharmacopeia of the United States
- ^ Government of Canada: Natural Health Products Regulations (SOR / 2003-196)
- ↑ Natural Health Products Directorate (2007): Evidence for Homeopathic Medicines Guidance (PDF; 689 kB)
- ^ Federal Association of the Pharmaceutical Industry eV: Pharma data 2014 ( Memento from September 23, 2015 in the Internet Archive )
- ↑ Steffen de Sombre: Homeopathic Medicines 2014 Awareness, Use and Image Institute for Demoskopie Allensbach, Bonn 2014.
- ↑ The Availability of Homeopathic and Anthroposophic Medicinal Products in the EU Archivlink ( Memento from 23 September 2015 in the Internet Archive )
- ↑ Share tip - Boiron: A specialty from France , Wirtschaftswoche from February 4, 2012.
- ↑ Homeopathy market in India , the average price of a homeopathic medicine in India is about 1/10 of the price in Germany