Continuous distillation

The continuous distillation is a kind of distillation leading to the separation process belongs. In the case of continuous distillation, the starting mixture is fed continuously (i.e. without interruption) into the process and the separated fractions are also discharged in continuous streams.
Each fraction can contain one or more components (types of chemical compounds). When distilling petroleum or similar feedstock, each fraction contains many components with similar volatility and different properties. Although continuous distillation operation is also possible on a small scale or in the laboratory, continuous distillation is most often used in large industrial plants.
Working principle
Distillation basics
A distillation is a separation or partial separation of a liquid feed mixture into a plurality of components or fractions by selective vaporization ( evaporation ), and following it, condensing due to different boiling points of the components. The working temperature lies between the boiling points of the components to be separated. This creates at least two fractions, at least one of which is the volatile distillate fraction, which is evaporated and then cooled, being collected as vapor that has again condensed to form a liquid. The bottom fraction (the residual component), namely the residue that does not evaporate at the working temperature, remains in the output container.
Continuous distillation
The functional principle of a continuous distillation does not differ from that of a normal distillation. When a liquid mixture is brought to a boil, the composition of the vapor above the liquid differs from the composition of the liquid itself. When this vapor is then separated and condensed into a liquid, it will contain a higher proportion of the lower boiling component (s) of the original Mixture on. This is exactly the process that takes place in a distillation column that is used for continuous distillation. A mixture is heated and fed into the distillation column. When entering the column, the starting material begins to flow downwards, but part of it, namely the component (s) with the lower boiling point (with the lower boiling points), evaporates (evaporates) and rises (rise). The steam cools down as it rises. While one part continues to rise as vapor, the other part (which contains a higher proportion of the less volatile component) is already beginning to move downwards again.
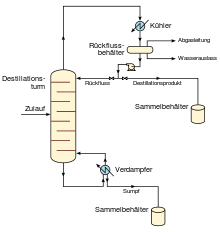
Figure 3 shows a simple fractionation column that is used for continuous distillation. In it, the output stream is separated into two fractions, namely into a top product and a bottom product. The “lightest” products (ie the products with the lowest boiling point or the most volatile products) exit from the top of the column and the “heaviest” products (the bottom, ie the products with the highest boiling point) exit from the bottom of the column out. The overhead fraction can be cooled and condensed with a water-cooled or air-cooled condenser. The sump evaporator can be a steam-heated or oil-heated heat exchanger or even a gas or oil-fired furnace.
In a continuous distillation, the system is kept in a steady state or an approximately steady state. In this context, a steady state means that the process-relevant quantities in operation do not change over time. These constant quantities include the amount of feedstock supplied, the amount of material flow dispensed , the heating rate and the cooling rate , the reflux ratio and the temperatures, pressures and compositions at each point in the process. If the process is not disturbed by changes in the amount of mixture supplied, the supplied thermal energy, the ambient temperature or with regard to condensation, the steady state normally remains. Apart from the minimal control effort (which, by the way, can easily be controlled by instruments), this is the main reason that makes continuous distillation so extremely attractive. If it is possible to keep the amount of the supplied starting mixture and the composition of the starting mixture constant, the product amount and product quality also remain constant. Even if there is a change in the state, modern process control methods are usually available to bring the continuous process back to the steady state.
A continuous distillation is often a fractional distillation ; it can be a vacuum distillation or a steam distillation .
Comparison with batch distillation
An alternative to continuous distillation is batch distillation , in which the mixture is added to the distillation plant at the beginning of the distillation, the distillate fractions are evaporated and condensed one after the other during the distillation and the remaining bottom fraction is removed at the end. Since each distillate fraction is evaporated and condensed here at different times, a single distillate outlet ( exit point ) is sufficient for batch distillation - the distillate can simply be switched to another collection vessel, a fraction collection container. Batch distillation is often used when smaller quantities are distilled. In contrast to batch distillation, with continuous distillation each fraction stream goes through the process simultaneously. Therefore, a separate distillate drain is required for each fraction. If there are several distillate fractions, in practice each distillate outlet is at a different height along the fractionation column . The bottom fraction can be drained from the bottom of the distillation column or system, but is often taken from a reboiler connected to the bottom of the column .
Since in a continuous distillation system, in contrast to batch distillation, the starting mixture is supplied in a constant amount and not all at once, no large distillation vessel or a container for a batch filling is required for continuous distillation. The mixture can therefore be introduced directly into the column in which the separation itself takes place. The height of the feed opening on the column may vary depending on the circumstances and it is designed to give the best results.
Design and operation
The design and operation of a distillation column depend on the starting material and the target products. In the case of a simple starting material with two components, analytical methods such as the McCabe-Thiele method or the Fenske equation can serve as an aid to the design. In the case of a starting material with many different components, computerized simulation models are used both for the design and subsequently for the operation of the column. Using models, columns that have already been installed can also be optimized for the distillation of mixtures for which they were not originally intended.
A column used for continuous distillation must be precisely controlled during operation so that changes in the composition of the starting material, the operating temperature and the product composition can be recognized. Many of these tasks are accomplished with advanced computer control equipment .
Material feed into the column
There are various options for feeding starting material into the column. If the material is supplied from a source whose pressure is higher than that of the distillation column, it can simply be introduced into the column via a pipe. Otherwise, the starting material is pumped into the column or compressed into it. The feedstock can be superheated vapor, saturated vapor, a partially vaporized liquid-vapor mixture, saturated liquid (i.e. the liquid is boiling at the pressure prevailing in the column) or supercooled liquid. If the starting material is a liquid, the pressure of which by far exceeds the column pressure, and if it flows through a pressure reducing valve directly in front of the column, there is immediate expansion and partial expansion evaporation, so that a liquid-vapor mixture is created when entering the distillation column .
Reflux
In large industrial fractionation columns, the product separation efficiency of the products is increased by the reflux. The reflux is that portion of the condensed, liquid top product from a distillation column that is returned to the upper part of the column. In the column, the reflux liquid running down ensures cooling and partial condensation of the rising vapors, which increases the efficiency of the distillation column. The more reflux is available, the better the separation of the lower-boiling from the higher-boiling components of the starting material in the column. The ratio of the amount of reflux to the distillate withdrawal is called the reflux ratio.
If an equilibrium is achieved between heating with reboiler at the bottom of the column and cooling with condensed reflux at the top of the column, a temperature gradient (or a stepwise temperature difference) is maintained in the column along the vertical axis, which is good prerequisites for the fractionation of the starting mixture offers. Reflux streams in the middle of the column are referred to as recirculations or pump arounds.
By changing the reflux (in connection with changes in the feed and the product withdrawal), the separation efficiency of a continuous distillation column can also be improved during operation (in contrast to this, adding additional plates or trays or changing the packing would result in at least a considerable downtime entail).
Increase in separation efficiency
Although small units, generally made of glass, can be used in laboratories, industrial plants use large, vertical steel vessels called "distillation columns" or "separation towers". To increase the separation efficiency, the interior of the column is normally equipped with horizontal plates or trays, or the column is filled with random packings. The heat required in the distillation for evaporation and to compensate for heat losses is in most cases supplied from a reboiler at the bottom of the column. The purity of the overhead product can be improved by recycling a portion of the externally condensed overhead liquid as reflux. Depending on their purpose, liquid outlets can be provided at certain intervals on the vertical axis of the distillation columns.
Structure of the column
Slabs or floors
In distillation columns, various vapor and liquid contact processes ensure the required number of equilibrium stages. These elements are commonly referred to as "slabs" or "floors". A different temperature and a different pressure prevail on each of these plates or on each of these floors. The pressure and temperature are highest at the stage at the column bottom. If we move up step by step in the column, the pressure and the temperature decrease each time. The vapor-liquid equilibrium for each feedstock component in the column reacts specifically to the various pressure and temperature conditions of the particular stage. This means that each component sets a certain concentration in the vapor and liquid phase in each stage. This causes the components to separate. A more detailed, enlarged illustration of two soils can be seen in the article theoretical soil. The reboiler often serves as an additional level of equilibrium. If every tray or plate had an efficiency of 100%, then the number of trays required that would be required for a specific separation would correspond to the number of equilibrium stages or theoretical plates. However, this is only very rarely the case. Therefore, a distillation column requires more plates than the required number of theoretical vapor-liquid equilibrium stages.
Packing
To improve separation in a distillation column, however, packing material can also be used instead of trays. These offer the advantage of a lower pressure drop in the column (compared to plates or trays), which is an advantage when operating in a vacuum. If filler material is used instead of trays in a distillation column, the number of theoretical equilibrium stages required is first determined. Then, the Füllkörperhöhe which corresponds to a theoretical equilibrium stage - this is used as a plate height designated (height equivalent to a theoretical plate and HETP) - also determined. The total required packing height corresponds to the number of theoretical stages multiplied by the separation stage height.
This filling material can either be a packing bed such as. B. Raschigringe or structured sheet metal. The liquids tend to wet the surface of the packing, and the vapors pull over this wetted surface, where a mass transfer takes place. In contrast to conventional tray distillation, where each tray corresponds to a separate point of the vapor-liquid equilibrium, the vapor-liquid equilibrium curve in a filled column is continuous. For the model-based recording of filled columns, however, it is useful to calculate the number of theoretical trays in order to be able to specify the separation efficiency of the filled column in relation to more traditional trays. With differently shaped fillings, the surfaces and the empty space between the fillings are different. Both factors affect the filling's performance.
In addition to the shape and surface of the filling, the distribution of liquid and vapor entering the filled bed is another factor that influences the performance of packed beds or structured packing. The number of theoretical stages required to perform a particular separation is calculated from a specific vapor-liquid ratio. If the liquid and the vapor are not evenly distributed over the column surface on entry into the filled bed, the vapor-liquid ratio in the filled bed is incorrect and the required separation is not achieved; the filling does not seem to be working correctly and the height equivalent to a theoretical plate or HETP is greater than expected. However, the problem does not lie in the filling itself, but in the poor distribution of the liquids entering the filled bed. More often the problem lies in a maldistribution of the liquid than the vapor. The design of the liquid distributors used to introduce the starting material and the reflux into the filled bed is decisive for whether the filling achieves maximum efficiency . Refer to the references for procedures for assessing the effectiveness of a liquid distributor.
Head system
In Figures 4 and 5 it is assumed that a top fraction is completely condensed to a liquid by means of water or air cooling. In many cases, however, it is difficult to completely condense the top of the column. Then the reflux tank must have a gas outlet. In still other cases, the overhead fraction may contain water vapor, either because the feedstock contains some water or because some steam is injected into the distillation column (as in the case of petroleum distillation columns in oil refineries). If the distillate product is insoluble in water, there may be a condensed liquid distillate phase, a condensed water phase, and a non-condensable gas phase in the reflux tank. Then the reflux tank must also be equipped with a water outlet.
Industrial use
General
Distillation is one of the basic operations in process engineering . In the chemical process industries, continuous distillation is used extensively in particular when large quantities of liquids have to be distilled. Examples include natural gas processing , petrochemical production , tar processing , beer brewing , liquefied air separation , hydrocarbon solvent production, and similar industries, but it is most extensively used in petroleum refineries . With crude oil, a highly complex mixture with many different components to be separated is used as starting material in these refineries. The result of this process is not the separation into pure chemical compounds, just the separation into groups of components with a relatively narrow boiling point range, called fractions. The term fractional distillation or fractionation is derived from this term “fractions”. In view of the product requirements and from the point of view of economic efficiency, it is often not worthwhile to carry out a further separation of the components within these fractions.
Large, vertical, cylindrical columns, which are referred to as "separation towers" or "distillation columns", are usually used for industrial distillation. These columns have a diameter of about 65 centimeters to 11 meters and a height of about 6 to 60 meters and more.
oil
Crude oil contains several hundred different hydrocarbon compounds: paraffins , naphthenes and aromatics, as well as organic sulfur compounds, organic nitrogen compounds and some oxygen-containing hydrocarbons such as phenols . Although crude oils do not normally contain olefins , they are formed in many of the processes used in a petroleum refinery.
The petroleum fractionator does not produce products that have a single boiling point, but fractions with boiling ranges. For example, the petroleum fractionator produces a top fraction known as “ naphtha ”, which, after further processing by a catalytic hydrogen desulfurizer to remove sulfur and a catalytic reformer to convert its hydrocarbon molecules into more complex molecules with a higher octane number , becomes a component of gasoline .
The so-called naphtha fraction contains many different hydrocarbon compounds. Therefore, it has a first boiling point of about 35 ° C and a final boiling point of about 200 ° C. Each fraction formed in the fractionation columns has a different boiling range. Below the top fraction, the next fraction is removed from the side of the column at a certain distance - normally this is the jet fuel fraction, which is also known as the kerosene fraction . The boiling range of this fraction extends from a first boiling point of about 150 ° C to a final boiling point of about 270 ° C, and it also contains many different hydrocarbons. The next fraction in the column (in descending direction) is the diesel oil fraction with a boiling range from 180 ° C to about 315 ° C. The boiling range between one fraction and the next fraction overlap, as the separations during distillation do not represent a perfectly sharp line. The next fraction is the heavy oil fraction , and finally there is the bottom fraction, whose boiling ranges are very broad. All of these fractions are subjected to further processing steps in downstream refinery processes.
Individual evidence
- ↑ a b c d Perry, Robert H. and Green, Don W .: Perry's Chemical Engineers' Handbook , 6th. Edition, McGraw-Hill, 1984, ISBN 0-07-049479-7 .
- ↑ Milton Beychok: Algebraic Solution of McCabe-Thiele Diagram . In: Chemical Engineering Progress . May 1951.
- ↑ Seader, JD, and Henley, Ernest J .: Separation Process Principles . Wiley, New York 1998, ISBN 0-471-58626-9 .
- ↑ a b Kister, Henry Z .: Distillation Design , 1st. Edition, McGraw-Hill, 1992, ISBN 0-07-034909-6 .
- ↑ Photographs of bubble cap and other tray types (website of Raschig GmbH).
- ↑ Random Packing, Vapor and Liquid Distribution: Liquid and gas distribution in commercial packed towers , Moore, F., Rukovena, F., Chemical Plants & Processing, Edition Europe, August 1987, pp. 11-15.
- ↑ Structured Packing, Liquid Distribution: A new method to assess liquid distributor quality , Spiegel, L., Chemical Engineering and Processing 45 (2006), 1011-1017.
- ↑ Editors: Jacqueline I. Kroschwitz and Arza Seidel: Kirk-Othmer Encyclopedia of Chemical Technology , 5th. Edition, Wiley-Interscience, Hoboken, NJ 2004, ISBN 0-471-48810-0 .
- ^ McCabe, W., Smith, J. and Harriott, P .: Unit Operations of Chemical Engineering , 7th. Edition, McGraw Hill, 2004, ISBN 0-07-284823-5 .
- ^ King, CJ: Separation Processes , 2nd. Edition, McGraw Hill, 1980, ISBN 0-07-034612-7 .
- ^ A b Gary, JH and Handwerk, GE: Petroleum Refining Technology and Economics , 2nd. Edition, Marcel Dekker, Inc., 1984, ISBN 0-8247-7150-8 .
- ^ Nelson, WL: Petroleum Refinery Engineering , 4th. Edition, McGraw Hill, 1958, LCCN 57010913.
Web links
- Distillation Theory (PDF; 184 kB) by Ivar J. Halvorsen and Sigurd Skogestad, Norwegian University of Science and Technology, Norway.
- Distillation, An Introduction by Ming Tham, Newcastle University, UK.
- Distillation by the Distillation Group, USA.
- Distillation Lecture Notes by Prof. Randall M. Price at Christian Brothers University.
- Petroleum Distillation by Wayne Pafco.
- Distillation simulation software .