Lithium borosilicide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
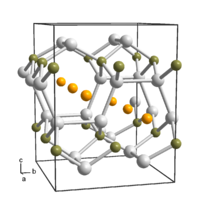
|
|||||||
| __ Li + __ B __ Si | |||||||
| General | |||||||
| Surname | Lithium borosilicide | ||||||
| other names |
tum |
||||||
| Ratio formula | LiBSi 2 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 73.923 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Lithium borosilicide is a chemical compound of lithium , silicon and boron with the empirical formula LiBSi 2 . The material was developed and manufactured in 2013 at the Technical University of Munich in cooperation with the Faculty of Physics at the University of Augsburg , the Department of Materials and Environmental Chemistry at the University of Stockholm and the high pressure laboratory of the Department of Chemistry and Biochemistry at Arizona State University . The new topology of the B-Si network was named tum after the Technical University of Munich .
Manufacturing
For the synthesis are lithium boride and silicon at a pressure of 100,000 atmospheres and 10 gigapascals and temperatures around 900 ° C reacted.
properties
Comparable to the carbon - atoms in a diamond , the boron and silicon atoms in the Lithiumborsilicid are (LiBSi 2 ) connected tetrahedral shape with one another. In addition, however, further channels are formed that allow lithium to be stored and removed again.
Lithium borosilicide is stable to air and moisture and can withstand temperatures of up to 800 ° C.
use
The material was developed as an alternative and more powerful material for an anode of lithium-ion accumulators . The usability is currently being investigated in more detail. Theoretically, the energy density of a lithium-ion battery can be increased by up to 25 percent by improving the anode.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ M. Zeilinger, L. van Wüllen, D. Benson, V. F. Kranak, S. Konar, T. F. Fässler, U. Häussermann: LiBSi 2 : A Tetrahedral Semiconductor Framework from Boron and Silicon Atoms Bearing Lithium Atoms in the Channels . In: Angewandte Chemie , 2013 , 125 (23) , pp. 6094-6098 doi : 10.1002 / anie.201301540
- ↑ Press release "Very promising material for lithium-ion batteries" , on www.tum.de; Retrieved June 7, 2013.
- ↑ Article "» tum «instead of graphite" , on www.elektroniknet.de; Retrieved June 7, 2013.