Pyruvate kinase M2
| Pyruvate kinase isoform M2 | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 530 amino acids | |
| Secondary to quaternary structure | Homodimer, homotetramer | |
| Cofactor | Magnesium, potassium | |
| Isoforms | L, R, M1, M2 | |
| Identifier | ||
| Gene name | PKM2 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 2.7.1.40 , kinase | |
| Response type | Phosphorylation | |
| Substrate | ADP + phosphoenolpyruvate | |
| Products | ATP + pyruvate | |
| Occurrence | ||
| Homology family | Pyruvate kinase | |
| Parent taxon | Euteleostomi | |
Pyruvate kinase M2 ( PKM2 , also: M2-PK or Tumor-M2-PK ) is an enzyme in humans that is only found in fetal and tumor tissue and therefore serves as a tumor marker . It is an isoform of pyruvate kinase called M2. The pyruvate kinase M2 is a key enzyme in tumor metabolism and can be used for therapy and monitoring of various tumor diseases. The pyruvate kinase M2 is not organ-specific tumor markers such as PSA , but the content of pyruvate kinase M2 reflected in the chair or EDTA - blood plasma as a biomarker specific metabolic state of the tumors.
Function of pyruvate kinase M2 in tumor metabolism
The pyruvate kinase catalyzes the last step within glycolysis , the dephosphorylation of phosphoenolpyruvate to pyruvate and is responsible for the net energy production in this metabolic pathway.
Depending on the different metabolic tasks of the various tissues, different isoenzymes of pyruvate kinase are expressed .
The M2-PK is the characteristic pyruvate kinase isoenzyme of all dividing cells. These include physiological cells such as B. fibroblasts, embryonic cells or adult stem cells , but also tumor cells.
As the tumor develops, there is a change in the isoenzyme pattern of the cells, whereby the respective tissue-specific isoenzyme, such as L-PK in the liver or M1-PK in the brain, disappears and the isoenzyme type M2 is increased.
Significance of the tetrameric and dimeric forms of pyruvate kinase M2 in tumor metabolism
M2-PK can occur in two different forms in proliferating cells:
- in a tetrameric form, which is composed of four subunits and
- in a dimeric form consisting of two subunits.
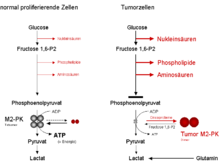
The tetrameric form of M2-PK has a high affinity for the substrate phosphoenolpyruvate and is highly active under physiological conditions. Furthermore, the tetrameric form is associated with other enzymes in the cytosol in the so-called glycolysis-enzyme complex, which enables a highly effective conversion of glucose into pyruvate and lactate due to the spatial proximity of the enzymes to one another.
If the M2-PK is predominantly in the highly active tetrameric form, as is the case in most normally proliferating cells, the glucose is mainly broken down into pyruvate with energy production.
The dimeric form of M2-PK has a low affinity for the substrate and is almost inactive under physiological conditions. The dimeric form is not associated in the glycolysis-enzyme complex.
If the M2-PK is predominantly in the less active dimeric form, as is the case in tumor cells, there is a backlog of all glycolysis intermediates above the pyruvate kinase reaction. These are available to the cells as starting materials for synthesis processes branching off from glycolysis. These synthetic pathways include nucleic acid, phospholipid and amino acid synthesis.
Nucleic acids , phospholipids and amino acids are important cell building blocks that cells that are highly active in dividing, such as tumor cells, urgently need.
Due to the key position of pyruvate kinase as the last enzyme within glycolysis, the tetramer: dimer ratio of the M2-PK decides whether the glucose carbon atoms are broken down into pyruvate and lactate while generating energy (tetrameric form) or are channeled into synthetic pathways.
Since the dimeric form of M2-PK generally predominates in tumor cells, the dimeric form of M2-PK was referred to as Tumor-M2-PK .
The dimerization of the M2-PK is induced in tumor cells by direct interaction of the M2-PK with various oncoproteins.
However, the ratio of tetramer to dimer of M2-PK in tumor cells is not a static state. Oxygen deficiency or highly pent-up metabolic intermediates, such as the glycolysis intermediate fructose-1,6-P2 or the amino acid serine , induce the reassociation of the dimeric form of M2-PK to the tetrameric form. As a result, through the reactivation of the M2-PK, the glucose is broken down into pyruvate and lactate with energy production until the fructose-1,6-P2 level falls below a certain limit and the M2-PK dissociates again into the dimeric form. The oscillation cycle starts again when the fructose-1,6-P2 levels rise above their signal level again and induce the tetramerization of the M2-PK.
If the M2-PK is predominantly in the less active dimeric form, the energy can be provided through the breakdown of the amino acid glutamine to aspartate, pyruvate and lactate (= glutaminolysis ).
The increased formation of lactate in the presence of oxygen observed in tumor cells is known as the Warburg effect .
Laboratory test
Pyruvate kinase M2 screening for the early detection of colorectal tumors and polyps
The M2-PK test is a stool examination for the early detection of colon cancer . Determination in the stool is carried out using ELISA technology in the laboratory or using a lateral flow test in the doctor's office. Positive results should be clarified with a colonoscopy . The test has been available in Germany as an Individual Health Service (IGeL) since 2007 . The IGeL monitor of the MDS (Medical Service of the Central Association of Health Insurance Funds) has analyzed the scientific literature on the M2-PK test and rates this self-pay service as “unclear”. There is no evidence that the M2-PK test for the early detection of colon cancer detects more tumors and advanced adenomas and correctly classifies more people as healthy than the blood stool test. The IGeL monitor therefore sees no evidence of a benefit from the M2-PK test if it is used in addition to or as an alternative to the blood stool test, but also no evidence of harm. The medical recommendation for action (guideline “Colorectal Carcinoma”) from 2017 also does not recommend the M2-PK test for colorectal cancer screening or early detection.
The probability of detecting bowel cancer with an M2-PK test ( sensitivity ) is 80.3% with the ELISA (60–100% depending on the tumor size ), the specificity (correct exclusion of a tumor) is 93%. However, there is criticism: 9 out of 10 sick people are recognized by the test. However, 247 out of 990 healthy test persons (25%) also get a false positive test result. Only 9 of 256 tested positive (3.5%) are therefore actually ill. (For a group of 1000 test persons.) Because of the poor specificity (82%), the detection of M2-PK in the stool is not suitable for screening examinations. The low specificity values shown in isolated studies can, as has meanwhile been shown, essentially be attributed to the fact that the study design was afflicted with serious methodological deficiencies. For example, people who were already ill were included in the relevant studies. In order to evaluate a screening method, however, it is essential to include only symptom-free people in the study in order to avoid falsification of the test results due to various previous illnesses of the test subjects. In August 2012, a meta-analysis was published in which 17 studies with a total of over 11,000 asymptomatic subjects, more than 700 people with colon cancer and over 500 people with polyps were comprehensively evaluated. The authors see the superiority of the biomarker as being based on the fact that it enables direct conclusions to be drawn about tumor metabolism and see the particular advantage of the M2-PK test in the fact that the test detects both bleeding and non-bleeding tumors and adenomas. In summary, the authors come to the conclusion that the M2-PK test, both as an ELISA and as a lateral flow rapid test, is a cost-effective and easy to perform routine test and end the analysis with the conclusion that the test for screening for colorectal cancer is unrestricted is recommend.
Depending on the study, the sensitivity of the lateral flow test is 85% (65–96%) for colon cancer and 56% (41–74%) for rectal cancer , and together for colorectal cancer it is 81.1% a specificity of 79% (76–81%) to 86.7%. This makes the M2-PK test more accurate than the tests for blood in stool ( guaiac test ) currently reimbursed by the statutory health insurance companies in Germany , which only detect colorectal cancer by approx. 20–40% (sensitivity) with a specificity of 92.2 %. A meta-analysis published in August 2012 with 11,000 asymptomatic test subjects showed a sensitivity for colorectal cancer of 80.3% and a specificity of 95.2%.
Pyruvate kinase M2 measurements for therapy and progress control in various tumor diseases
Studies international working groups have shown that the content of M2-PK in EDTA - plasma of patients with renal, lung, breast, cervical tumors, tumors of the gastrointestinal tract (esophagus, stomach, pancreas, colon and rectum) as well as in malignant melanoma with correlated with the stage of the tumors.
An important area of application for the detection of M2-PK in EDTA plasma are therefore follow-up controls during therapy, which enable the success or failure of a therapy to be recognized at an early stage. If the M2-PK values in the EDTA plasma of the tumor patient fall during the therapy and if they remain permanently low, this indicates a good success of the therapy. If the M2-PK values increase during or after therapy, this is an indication that a relapse and / or metastases have formed. Elevated M2-PK values can also occur in inflammatory diseases and must be excluded from the differential diagnosis.
Individual evidence
- ↑ IGeL-Monitor, evaluation of autologous blood therapy for tendinopathy . Retrieved October 8, 2018.
- ↑ Guideline (Recommended Action for Doctors) " Colorectal Carcinoma " of November 30, 2017
- ↑ a b c d e C. Tonus, M. Sellinger u. a .: Faecal pyruvate kinase isoenzyme type M2 for colorectal cancer screening: A meta-analysis. ( Memento of the original from October 9, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 937 kB) In: World journal of gastroenterology: WJG. Volume 18, Number 30, August 2012, pp. 4004-4011, ISSN 1007-9327 . doi: 10.3748 / wjg.v18.i30.4004 . PMID 22912551 . PMC 3419997 (free full text).
- ^ A b C. Tonus, G. Neupert, M. Sellinger: Colorectal cancer screening by non-invasive metabolic biomarker fecal tumor M2-PK. (PDF; 789 kB) In: World journal of gastroenterology: WJG. Volume 12, Number 43, November 2006, pp. 7007-7011, ISSN 1007-9327 . PMID 17109496 .
- ↑ arznei-telegram 6/2007: ENZYME TEST "M2-PK" SUPERIOR SCREENING MARKER FOR COLON CANCER?
- ↑ U. Haug, S. Hundt, H. Brenner: Sensitivity and specificity of faecal tumor M2 pyruvate kinase for detection of colorectal adenomas in a large screening study. In: Br J Cancer. 99 (1), 2008 Jul 8, pp. 133-135. Epub 2008 Jun 10 PMID 18542075 .
- ↑ a b U. Haug, D. Rothenbacher u. a .: Tumour M2-PK as a stool marker for colorectal cancer: comparative analysis in a large sample of unselected older adults vs colorectal cancer patients. In: British journal of cancer . Volume 96, Number 9, May 2007, pp. 1329-1334, ISSN 0007-0920 . doi: 10.1038 / sj.bjc.6603712 . PMID 17406361 . PMC 2360192 (free full text).
- ↑ a b c Y. M. Shastri, M. Naumann u. a .: Prospective multicenter evaluation of fecal tumor pyruvate kinase type M2 (M2-PK) as a screening biomarker for colorectal neoplasia. In: Journal international du cancer. Volume 119, Number 11, December 2006, pp. 2651-2656, ISSN 0020-7136 . doi: 10.1002 / ijc.22243 . PMID 16929517 .
- ↑ JE Allison, IS Tekawa, LJ Ransom, AL Adrain: A comparison of fecal occult-blood testing for colorectal-cancer screening. In: N Engl J Med . 334 (3), 1996 Jan 18, pp. 155-159. PMID 8531970 .
- ^ DA Lieberman, DG Weiss; Veterans Affairs Cooperative Study Group 380: One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. In: N Engl J Med. 345 (8), 2001 Aug 23, pp. 555-560. PMID 11529208
- ↑ Yogesh Kumar, Niteen Tapuria, Naveed Kirmani, Brian R. Davidson: Tumor M2-pyruvate kinase: a gastrointestinal cancer marker. In: European Journal of Gastroenterology & Hepatology. 19, 2007, pp. 265-276, doi: 10.1097 / MEG.0b013e3280102f78 .
- ↑ PD Hardt, BK Ngoumou, J. Rupp, H. Schnell-Kretschmer, HU Kloer: Tumor M2-pyruvate kinase: a promising tumor marker in the diagnosis of gastrointestinal cancer. In: Anticancer Res . 20 (6D), 2000 Nov-Dec, pp. 4965-4968. PMID 11326648 .
- ↑ DK Dhar, SW Olde Damink, JH Brindley, A. Godfrey, MH Chapman, NS Sandanayake, F. Andreola, S. Mazurek, T. Hasan, M. Malago, SP Pereira: Pyruvate kinase M2 is a novel diagnostic marker and predicts tumor progression in human biliary tract cancer. In: Cancer . Volume 119, Number 3, February 2013, pp. 575-585, ISSN 1097-0142 . doi: 10.1002 / cncr.27611 . PMID 22864959 . PMC 3492546 (free full text).
further reading
Pyruvate kinase M2 in stool
- PD Hardt, S. Mazurek, M. Toepler, P. Schlierbach, RG Bretzel, E. Eigenbrodt, HU Kloer: Faecal tumor M2 pyruvate kinase: a new, sensitive screening tool for colorectal cancer. In: Brit. J. Cancer. 91, 2004, pp. 980-984. PMID 15266315
- K. Koss, D. Maxton, JAZ Jankowski: The potential use of fecal dimeric M2 pyruvate kinase (Tumor M2-PK) in screening for colorectal cancer (CRC). Abstract from Digestive Disease Week. May 2005; Chicago, USA.
- R. Mc Loughlin, E. Shiel, S. Sebastian, B. Ryan, HJ O'Connor, C. O'Morain: Tumor M2-PK, a novel screening tool for colorectal cancer. Abstract from Digestive Disease Week. May 2005, Chicago / USA
- C. Tonus, G. Neupert, M. Sellinger: Colorectal cancer screening by non-invasive metabolic biomarker fecal M2-PK. In: World J Gastroenterol. 12, 2006, pp. 7007-7011. PMID 17109496
- C. Tonus, G. Neupert, K. Witzel: The faecal tumor M2-PK screening test for invasive & pre-invasive colorectal cancer: estimated specificity & results as a function of age for a study population of 4854 volunteers. . In: Nowotwory J Oncol. 59, 2009, pp. 32e-37e.
Pyruvate kinase M2 in plasma
- H. Cerwenka, R. Aigner, H. Bacher, G. Werkgartner, A. El-Shabrawi, F. Quehenberger, HJ Mischinger: TUM2-PK (pyruvate kinase type tumor M2), CA19-9 and CEA in patients with benign, malignant and metastasizing pancreatic lesions. In: Anticancer Res. 19, 1999, pp. 849-852. PMID 10216504
- B. Kaura, R. Bagga, FD Patel: Evaluation of the pyruvate kinase isoenzyme tumor (Tu M2-PK) as a tumor marker for cervical carcinoma. In: J. Obstet. Gynaecol. Res. 30, 2004, pp. 193-196. PMID 15210041
- CW Kim, JI Kim, SH Park, JY Han, JK Kim, KW Chung, HS Sun: Usefulness of plasma tumor M2-pyruvate kinase in the diagnosis of gastrointestinal cancer. In: Korean J. Gastroenterol. 42, 2003, pp. 387-393. PMID 14646575
- D. Lüftner, J. Mesterharm, C. Akrivakis, R. Geppert, PE Petrides, KD Wernecke, K. Possinger: Tumor M2-pyruvate kinase expression in advanced breast cancer. In: Anticancer Res. 20, 2000, pp. 5077-5082. PMID 11326672 .
- GM Oremek, S. Teigelkamp, W. Kramer, E. Eigenbrodt, KH Usadel: The pyruvate kinase isoenzyme tumor M2 (Tu M2-PK) as a tumor marker for renal carcinoma. In: Anticancer Res. 19, 1999, pp. 2599-2601. PMID 10470201
- J. Schneider, H. Morr, HG Velcovsky, G. Weisse, E. Eigenbrodt: Quantitative detection of tumor M2-pyruvate kinase in plasma of patients with lung cancer in comparison to other lung diseases. In: Cancer Detec. Prev. 24, 2000, pp. 531-535. PMID 11198266
- J. Schneider, G. Schulze: Comparison of Tumor M2-pyruvate kinase (Tumor M2-PK), carcinoembryonic antigen (CEA), carbohydrate antigens CA 19-9 and CA 724 in the diagnosis of gastrointestinal cancer. In: Anticancer Res. 23, 2003, pp. 5089-5095. PMID 14981971
- S. Ugurel, N. Bell, A. Sucker, A. Zimpfer, W. Rittgen, D. Schadendorf: Tumor type M2 pyruvate kinase (TuM2-PK) as a novel plasma tumor marker in melanoma. In: Int. J. Cancer. 117, 2005, pp. 825-830. PMID 15957165
- M. Ventrucci, A. Cipolla, C. Racchini, R. Casadei, P. Simoni, L. Gullo: Tumor M2-pyruvate kinase, a new metabolic marker for pancreatic cancer. In: Dig. Dis. Sci. 49, 2004, pp. 1149-1155. PMID 15387337
- HW Wechsel, E. Petri, KH Bichler, G. Feil: Marker for renal carcinoma (RCC): The dimeric form of pyruvate kinase type M2 (Tu M2-PK). In: Anticancer Res. 19, 1999, pp. 2583-2590. PMID 10470199
- B. Zhang, JY Chen, DD. Chen, GB. Wang, P. Shen: Tumor type M2 pyruvate kinase epxression in gastric cancer, colorectal cancer and controls. In: World J. Gastroenterol. 10, 2004, pp. 1643-1646. PMID 15162541
Scientific background on pyruvate kinase M2
- S. Mazurek, CB Boschek, F. Hugo, E. Eigenbrodt: Pyruvate kinase type M2 and its role in tumor growth and spreading. In: Semin. Cancer Biol. 15, 2005, pp. 300-308. PMID 15908230