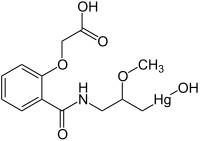
Mersalyl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Mersalyl | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 17 HgNO 6 | ||||||||||||||||||
| Brief description |
White dust |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 483.87 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.043 |
||||||||||||||||||
| Melting point |
192–193 ° C (decomposition) |
||||||||||||||||||
| solubility |
soluble in ammonia, |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Mersalyl (actually Mersalylic Acid ) is an organomercury compound with a diuretic effect. Mersalyl is no longer used as a drug today as it has been supplanted by other diuretics that do not contain mercury and are therefore less toxic .
Mersalyl has been used in biochemical experiments since the 1960s as a water-soluble, non-membrane-permeable inhibitor for the reversible blocking of sulfhydryl groups in proteins .
synthesis
The first synthesis of mersalyl (sodium salt, 505.87 g / mol) was published by Otto Diels and Erich Beccard . The taste is described as bitter . When Mersalyl is exposed to daylight or is in solution, the compound slowly decomposes and releases mercury; For this reason, small amounts of theophylline are often added to the substance , which slow down this process.
Bockmühl and Schwarz applied for a patent on Mersalyl for Hoechst in 1925 .
Individual evidence
- ↑ a b c Mersalyl acid data sheet (PDF; 274 kB) from Santa Cruz Biotechnology, accessed on April 13, 2012.
- ↑ a b c d data sheet Mersalyl acid, analytical standard at Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ a b E. Brown Robbins, K. K Chen: A new mercurial diuretic . In: Journal of the American Pharmaceutical Association . tape 40 , no. 5 , May 1951, p. 249-251 , doi : 10.1002 / jps.3030400509 .
- ↑ Otto Diels, Erich Beccard: On the knowledge of acylated allylamines . In: Reports of the German Chemical Society . tape 39 , no. 4 , 1906, pp. 4125-4132 , doi : 10.1002 / cber.190603904108 .
- ↑ Patent DE423031 : Published January 6, 1926 .
literature
- The Merck Index (1983), No. 5750 (p. 843)