Mesuximide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
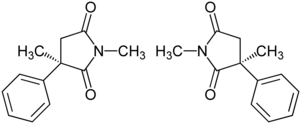
|
||||||||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Mesuximide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 12 H 13 NO 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 203.2 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Mesuximide ( N, 2-dimethyl-2-phenyl-suximide , trade name Petinutin ® ) is an antispasmodic drug from the group of anticonvulsants that is used for the long-term treatment of certain forms of epilepsy . Chemically, it belongs to the group of the Suximide and is rapidly and almost completely converted to its active metabolite N -desmethylmesuximide.
application areas
Mesuximide is only used as a second line drug in otherwise treatment-resistant epilepsy . It is used as an additional medication in particular for absences and epilepsy with complex focal seizures, but also for Lennox-Gastaut syndrome .
Side effects
Depending on the dose, there is a lack of appetite, nausea, vomiting, hiccups, abdominal pain, fatigue, headache, photophobia, confusion, imbalance, dizziness and, rarely, a temporary reduction in white blood cells . Irrespective of the dose, allergic skin reactions, behavior changes and involuntary movement disorders can occur.
chemistry
The active ingredient is synthesized by a method described in the literature, starting from acetophenone and methyl cyanoacetate . Mesuximide is used as a racemic active ingredient.
Trade names
Mesuximide is commercially available in Germany and Switzerland under the name Petinutin.
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of Mesuximide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on February 3, 2020, is reproduced from a self-classification by the distributor .
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dieter Reichert: Pharmaceutical Substances . Syntheses, Patents and Applications of the most relevant APIs. 5th edition. Thieme-Verlag, Stuttgart, New York 2009, ISBN 978-3-13-558405-8 , pp. 860 ( limited preview in Google Book search).