N -alkyl-β-aminopropionic acids
N- alkyl-β-aminopropionic acids are derivatives of the non-proteinogenic amino acid β-alanine , in which the nitrogen atom usuallycarriesa longer (≥ C 8 ) unbranched or branched alkyl group C n H 2n + 1 . In industrial synthesis, N -alkyl-β-aminopropionic acids are always formed together with N -alkyliminodipropionic acids. Because of the amino group and the carboxyl group in the molecule, N- alkyl-β-alanines are zwitterions and because of the long alkyl chain amphoteric surfactants with a wide variety of cosmetic and technical applications. Other amphoteric surfactants with zwitterionic properties are betaines , sulfobetaines , amine oxides , amphoacetates and amphodiacetates, such as. B. the disodium cocoamphodiacetate .
Manufacturing
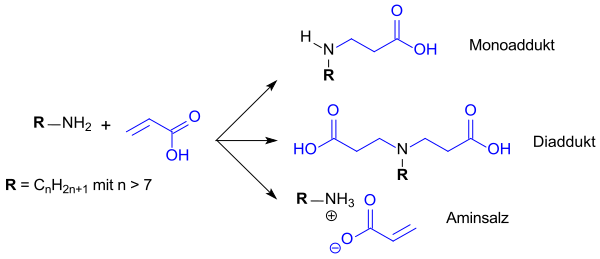
The synthesis of “new aminocarboxylic acids” first described by Walter Reppe at IG Farben AG in 1938 involves a Michael addition of acrylic acid to a longer-chain amine, e.g. B. dodecylamine . The Reppe patent describes the formation of N -dodecyl-β-aminopropionic acid with the addition of aqueous acrylic acid to excess dodecylamine, and with excess acrylic acid the formation of N -dodecyliminodipropionic acid or its sodium salts after neutralization with sodium carbonate . However, there is a lack of analytical data on the end products.
Depending on the input factors of the starting materials and the process conditions, a mixture of aminopropionic acid (monoaddition product), iminodipropionic acid (diaddition product) and the amine salt with acrylic acid (starting materials) is always obtained in industrial synthesis. Longer-chain amines (> C 14 ), higher temperatures (> 150 ° C) and longer reaction times (> 10 h) shift the yields towards more monoproduct when the amounts used are approximately equimolar. However, these conditions are uneconomical and lead to undesired yellowing of the batch and to thermally induced polymerization of acrylic acid. In addition to the relatively undefined product composition, the disadvantage is the content of unreacted amine and acrylic acid, which leads to odor nuisance and skin irritation and is particularly useful in cosmetic applications, e.g. B. in shampoos, cannot be tolerated.
The inconsistency of the product mixtures obtained in the industrial synthesis of N -alkyl-β-alanines has set in motion efforts to arrive at better-defined products by changing the reactants and the process conditions. Instead of acrylic acid, short-chain acrylic acid esters , usually the pungent-smelling methyl acrylate , or catalysts for the Michael addition were used, which although lower reaction temperatures, e.g. B. allow 30 ° C and shorter reaction times , but one or two additional process steps - hydrolysis of the N -alkyl-β-alanine ester with alkali metal hydroxides and subsequent acidification - to represent the acid.
Systematic studies with the reactants laurylamine or cocoalkylamine and acrylic acid show that only slightly different product compositions are obtained by varying the pH value during the reaction. Typically, with cocoalkylamine at pH ≈ 6, predominantly the monoadduct N- cocoalkylaminopropionic acid (45% monoadduct, 28% diadduct, 27% amine salt), at pH ≈ 4.5 predominantly the diadduct (38% mono-, 41% diadduct, 21% amine salt ) educated.
For technical applications, the subsequent separation of the aminopropionic acid / iminodipropionic acid mixture, e.g. B. by flash chromatography , not. The amine salts, on the other hand, can simply be crystallized out, so that salt-free solutions are obtained.
properties
The mixture of substances obtained in the industrial synthesis of N- alkyl-β-aminopropionic acids is diluted with water to a solids content of approx. 30 to 50% and usually results in clear, yellow solutions, the pH of which is adjusted to 8 to 10.
N -Alkyl-β-alanines are little to non-foaming surfactants that are just as stable in acidic and alkaline media as in hard water or to high electrolyte concentrations, such as. B. in sea water. In contrast to most surfactants, the N- alkyl-β-aminopropionic acid mixtures , which are readily water-soluble over a wide pH range , are compatible with cationic , anionic and nonionic surfactants .
What is remarkable is the high hydrotropy of these mixtures, which reduces the viscosity of aqueous systems and, through their solubilizing effect, suppresses the phase separation of otherwise incompatible components. Iminodipropionates with two carboxyl groups are more hydrophilic than aminopropionic acids, in which only one acrylic acid residue is added to the amine in the Michael reaction.
These surfactants, in particular the diaddition product octyliminodipropionate (CAS No. 94441-92-6, EC No. 305-318-6), are also distinguished by a pronounced and lasting effectiveness as a corrosion protection agent .
At a basic pH value, the solutions are irritating to the skin and eyes, but are readily biodegradable and non-toxic under normal conditions.
use
N -Alkyl-β-aminopropionic acids have a wide range of uses, e.g. B .:
- as a hydrotrope to increase the solubility, in particular of nonionic surfactants, and the stability of the preparations obtained, even at high electrolyte concentrations
- as a solubilizer for cationic surfactants in cleaners with an antibacterial effect
- As a co-surfactant in surface cleaners (English hard surface cleaners ) for industrial and commercial purposes (English industrial and institutional cleaning, I&I ), e.g. B. also Cleaning in Place CIP in the food industry
- as a foam stabilizer in concentrated salt solutions, such as. B. in drilling fluids in oil exploration and production
- as a degreasing agent in highly alkaline cleaners in which most ionic and non-ionic emulsifiers are ineffective
- as a corrosion inhibitor in cooling lubricants for metalworking
Individual evidence
- ↑ Eric G. Lomax: Amphoteric Surfactants, 2nd Edition . Marcel Dekker, Inc., Nw York, NY, USA 1996, ISBN 0-8247-9392-7 .
- ↑ Patent US2195974 : Process of producing new aminocarboxylic acids. Registered on July 10, 1937 , published on April 2, 1940 , applicant: IG Farbenindustrie AG, inventor: W. Reppe, H. Ufer.
- ↑ J. Comelles, M. Moreno-Mañas, A. Vallribera: Michael additions catalyzed by transition metals and lanthanide species. A review . In: ARKIVOC . ix, 2005, p. 207-239 ( arkat-usa.org ).
- ↑ Patent US2468012 : Beta amino propionates. Filed August 6, 1945 , published April 19, 1949 , applicant: General Mills, Inc., inventor: AF Isbell.
- ↑ Patent WO9950227 : Process for the selective control of zwitterionic amphoteric surfactant compositions. Applied March 24, 1999 , published October 7, 1999 , applicant: Akzo Nobel NV, inventor: LJ Joffre.
- ^ J. Escalante, M. Carrillo-Morales, I. Lanzaga: Michael additions of amines to methyl acrylates promoted by microwave irridiation . In: Molecules . tape 13 , no. 2 , 2008, p. 340-347 , doi : 10.3390 / molecules13020340 .
- ↑ Ampholak YJH-40, Octyliminodipropionate. (PDF; 46 kB) In: surfacechemistry.nouryon.com. Nouryon, accessed October 12, 2019 .
- ↑ ColaRTeric ZF-50. (PDF; 189 kB) In: colonialchemical.com. Colonial Chemical, Inc., accessed October 12, 2019 .
- ↑ CL Burnett et al .: Safety Assessment of Lauriminodipropionic Acid, Sodium Lauriminodipropionate, and Disodium Lauriminodipropionate as Used in Cosmetics . In: Int. J. Toxicol. tape 32 , no. 3 , 2013, p. 49S-55S , doi : 10.1177 / 1091581813497765 .
- ↑ DeriphatR 160 C, Technical Bulletin. (PDF; 31 kB) BASF Corp., accessed on October 12, 2019 (English).
- ↑ FlexisurfTM EHDP, Technical Data Sheet. (PDF; 349 kB) Innovative Chemical Technologies, Inc., accessed on October 12, 2019 .