Naphthols
| Naphthols | ||
| Surname | 1-naphthol | 2-naphthol |
| other names | α-naphthol, 1-hydroxynaphthalene, naphth-1-ol |
β-naphthol, 2-hydroxynaphthalene, naphth-2-ol |
| Structural formula | 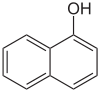 |

|
| CAS number | 90-15-3 | 135-19-3 |
| PubChem | 7005 | 8663 |
| Molecular formula | C 10 H 8 O | |
| Molar mass | 144.17 g mol −1 | |
| density | 1.28 g cm −3 | 1.22 g cm −3 |
| Physical state | firmly | |
| Brief description | colorless crystals with a faint phenolic odor |
white to yellowish crystalline powder with a phenolic odor |
| Melting point | 96 ° C | 123 ° C |
| boiling point | 288 ° C | 285 ° C |
| pK s value | 9.30 | 9.57 |
| solubility | 0.1 g l −1 (20 ° C) | 1 g l −1 (20 ° C) |
Naphthols are chemical substances that are also known as hydroxynaphthalenes because they are derivatives of naphthalene in which one or more hydrogen atoms are replaced by hydroxyl groups . The simplest naphthols are 1-naphthol (α-naphthol, 1-hydroxynaphthalene) and 2-naphthol (β-naphthol, 2-hydroxynaphthalene).
properties
Naphthols react chemically in a similar way to phenols , but are usually more reactive. Naphthols are difficult in cold water and more soluble in hot water. Since naphthols, like phenols, are weak acids, they are more soluble in dilute caustic soda than in water. Organic solvents such as ethanol , ether and benzene dissolve naphthols well.
use
The naphthols are often used for the synthesis of azo dyes , tanning agents, dyeing and printing auxiliaries, and preservatives for glue, wood and leather. So z. B. 2-naphthol coupled with diazotized 4-nitroaniline to form parrot :
 Synthesis of para-red starting from 4-nitroaniline ( 1 ). After the action of sulfuric acid and sodium nitrite, this reacts to form a diazonium salt ( 2 ), which is coupled with 2-naphthol to form parrot ( 3 ).
Synthesis of para-red starting from 4-nitroaniline ( 1 ). After the action of sulfuric acid and sodium nitrite, this reacts to form a diazonium salt ( 2 ), which is coupled with 2-naphthol to form parrot ( 3 ).
Individual evidence
- ↑ a b c d Entry for CAS no. 90-15-3 in the GESTIS substance database of the IFA , accessed on April 14, 2013(JavaScript required) .
- ↑ a b c d Entry for CAS no. 135-19-3 in the GESTIS substance database of the IFA , accessed on April 14, 2013(JavaScript required) .
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Toxicological assessment of 2-naphthol (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 20, 2012.
- ↑ JR Mohrig, TC Morrill, CN Hammond, DC Necker: Synthesis 5: Synthesis of the dye Para Red from aniline , in: Experimental Organic Chemistry Freeman: New York, NY, 1997; Pp. 456-467.