Sodium hexametaphosphate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
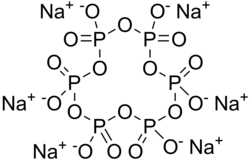
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium hexametaphosphate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | Na 6 O 18 P 6 | ||||||||||||||||||
| Brief description |
crystalline white odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 611.77 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.58 g cm −3 |
||||||||||||||||||
| Melting point |
550 ° C |
||||||||||||||||||
| boiling point |
1500 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.482 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Sodium hexametaphosphate is an inorganic chemical compound of sodium from the group of metaphosphates . The products of this name commercially available today are incorrectly not pure hexametaphosphate, but a mixture of different polyphosphates with a chain length of mainly 10 to 15 (in some cases down to 4), whereby carefully selected synthesis processes limit this to 11 to 13. The products sold under the trade names Calgon and Quadrofis had a chain length of mainly 10 to 20 and 4 to 8 respectively.
Extraction and presentation
Sodium hexametaphosphate can be obtained by rapidly cooling molten sodium trimetaphosphate. For pure cyclic sodium hexametaphosphate instead of the technical product can be obtained by fractionation.
properties
Sodium hexametaphosphate is a crystalline white odorless solid that is soluble in water.
use
Sodium hexametaphosphate is a food additive used in dairy products, canned foods, packaged egg whites, ice cream, seafood, and meat processing. It is also used as a sequestering agent, water softener, and cleaning agent. It is an active ingredient in toothpaste as an anti-stain agent. It prevents the corrosion of steel. It is also used in building and water treatment as well as in photographic development and as a retarder in dental alginate impression materials and as a thinner for petroleum drilling fluids.
Individual evidence
- ↑ a b c d e f g h Entry on sodium hexametaphosphate in the GESTIS substance database of the IFA , accessed on February 11, 2019(JavaScript required) .
- ↑ a b c d data sheet Sodium hexametaphosphate, tech. at AlfaAesar, accessed on February 11, 2019 ( PDF )(JavaScript required) .
- ↑ Thomas E. Furia: CRC Handbook of Food Additives, Second Edition . CRC Press, 1973, ISBN 978-0-8493-0542-9 , pp. 630 ( limited preview in Google Book search).
- ↑ Elizabeth S. Baluyot, Clark G. Hartford: Comparison of polyphosphate analysis by ion chromatography and by modified end-group titration. In: Journal of Chromatography A. 739, 1996, p. 217, doi : 10.1016 / 0021-9673 (96) 00027-1 .
- ↑ Entry on Sodium Hexametaphosphate in the Hazardous Substances Data Bank , accessed February 11, 2019.
- ^ S. Greenfield, M. Clift: Analytical Chemistry of the Condensed Phosphates . Elsevier, 2013, ISBN 978-1-4832-8065-3 , pp. 191 ( limited preview in Google Book search).