Nitrocefin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
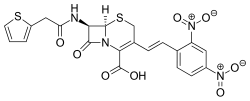
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Nitrocefin | |||||||||||||||||||||
| other names |
(7 R ) -3 - (( E ) -2,4-Dinitrostyryl) -7- (2-thienylacetamido) -3-cephem-4-carboxylic acid |
|||||||||||||||||||||
| Molecular formula | C 21 H 16 N 4 O 8 S 2 | |||||||||||||||||||||
| Brief description |
yellow-orange solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 516.5 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
167-169 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Nitrocefin is a substance belonging to the chemical group of the cephalosporins , which changes its color from yellow to red when split by β-lactamases .
Properties and use
The pure substance dissolves well in dimethyl sulfoxide . She is sensitive to light. It was first isolated from the Acremonium mold discovered by Giuseppe Brotzu .
Nitrocefin is a chromogenic cephalosporin : when the β- lactam ring is hydrolytically opened, the absorption maximum shifts from 390 nm to 486 nm, which is accompanied by a color change from yellow to red. Nitrocefin is therefore suitable for the detection of bacteria that produce β-lactamases. β-lactamases play an important role in the feared antibiotic resistance of bacteria. The test is more sensitive than the test previously carried out on the basis of bromocresol purple .
literature
- JP Richard: Advances in Physical Organic Chemistry , Volume 41, 2006, p. 117 ( limited preview in Google book search)
- Monica Cheesbrough: District Laboratory Practice in Tropical Countries , Volume 2, 2006, p. 142 ( limited preview in Google Book Search)
- Victor Lorian: Antibiotics in laboratory medicine , 2005, p. 485 ( limited preview in Google book search)
- Karin Hammann-Meyer: Investigations into the diagnostic value of Nitrocefin, 1981.
Individual evidence
- ↑ nugi center: Nitrocefin (PDF; 183 kB).
- ↑ Mijoon Lee, Dusan Hesek, Shahriar Mobashery: A Practical Synthesis of Nitrocefin. In: The Journal of Organic Chemistry. 70, 2005, pp. 367-369, doi : 10.1021 / jo0487395 .
- ↑ a b Data sheet Nitrocefin - CAS 41906-86-9 - Calbiochem, A chromogenic β-lactamase substrate that undergoes distinctive color change from yellow as the amide bond in the β-lactam ring is hydrolyzed by β-lactamase. at Sigma-Aldrich , accessed on December 21, 2019 ( PDF ).
- ^ GA Papanicolaou, AA Medeiros: Discrimination of extended-spectrum beta-lactamases by a novel nitrocefin competition assay. In: Antimicrobial agents and chemotherapy. Volume 34, Number 11, November 1990, pp. 2184-2192, PMID 2073109 , PMC 172021 (free full text).