Nitrofural
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
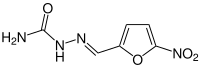
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Nitrofural | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 6 H 6 N 4 O 4 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 198.14 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
242-244 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Nitrofural (trade name Furacin) is a drug from the group of antibiotic nitrofurans . It is used in the local treatment of superficial infected skin diseases and wound infections caused by pathogenic nitrofural-sensitive bacteria.
application
With nitrofurazone-impregnated urinary catheters , the risk could reduce of catheter-associated urinary tract infections in adult patients. However, it is not used in patients with impaired renal function . Experience with the use in humans is available; the active ingredient is on the market in Germany under the trade name Furacin-SOL. The animal experiment produced indications of embryotoxic / teratogenic effects. Therefore, nitrofural should not be used in pregnant women. It is not known whether the substance is excreted in breast milk , so nitrofural should not be used during breastfeeding.
Side effects
Occasionally, allergic reactions with reddening of the skin and itching are observed.
Individual evidence
- ↑ a b c data sheet 5-Nitro-2-furaldehyde semicarbazone from Sigma-Aldrich , accessed on July 8, 2017 ( PDF ).
- ^ Smoot EC Susceptibility testing of topical antibacterials against methicillin-resistant Staphylococcus aureus. In: J Burn Care Rehabil . 1992 Mar-Apr; 13 (2 Pt 1): 198-202, PMID 1587917 .
- ↑ Evens F et al. Nitrofurazone therapy of Trypanosoma gambiense sleeping sickness in man. In: Am J Trop Med Hyg . 1957 Jul; 6 (4): 665-678, PMID 13458672 .
- ↑ Stensballe J et al. Infection Risk with Nitrofurazone-Impregnated Urinary Catheters in Trauma Patients: A Randomized Trial . In: Annals of Internal Medicine . 2007 Sep 4; 147 (5): 285-293, PMID 17785483 .
- ↑ Ballmer-Weber BK Contact allergy to nitrofurazone. In: Contact Dermatitis . 1994; 31 (4): 274-275, PMID 7842698 .
