Nitrofurans
| 2-nitrofuran and its derivatives |
|---|
|
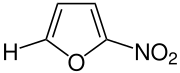
2-Nitrofuran with drawn hydrogen atom, which is replaced by other radicals in the derivatives. |
|
General structural formula of the nitrofurans ( R = organic radical, which can also contain heteroatoms). |
Nitrofurans are a group of chemical compounds consisting of the derivatives of 2-nitrofuran . There are numerous representatives, such as the nitrofurantoin used against urinary tract diseases (see below). Often they are named internationally with the prefix Nitrofuran or with their abbreviated forms Nifur and Fur . During the 1940s, the fungicidal and bactericidal effects of nitrofurans were discovered, which is why they are used in a variety of ways in these areas of application. The nitrofurans can be divided into antibiotics and microantibiotics according to their mode of action. Few representatives of the nitrofurans were used briefly for food preservation in East Asia, but this practice was discontinued due to mutagenic properties.
synthesis
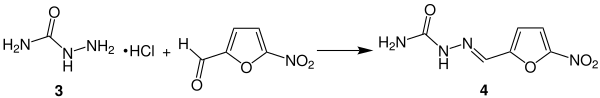
Starting from the industrially obtained from plant materials furfural ( 1 ) is first with the addition of nitrating acid in a regioselective electrophilic aromatic substitution of the 5-Nitrofurfurylaldehyd ( 2 prepared):
The nitrofuran 2 obtained from the nitration can now be further processed to a large part of the pharmaceutically relevant nitrofurans - if necessary after lengthening the side chain - with the addition of appropriate hydrazides or their salts . The simplest example of this is the synthesis of nitrofural ( 4 ) starting from the hydrochloride 3 :
Use as antibiotics
Most of the nitrofurans used as active ingredients can be classified under the antibiotic category.
Nitrofurantoin
| Structural formula with marked remainder and international non-proprietary name | Example of an application area |
|---|---|
 Nitrofurantoin trade names: Furadantin, Nifuretten, Uro-Tablinen |
 Control of Escherichia coli bacteria |
Nitrofurantoin is used to combat Escherichia coli bacteria, as a remedy for urinary tract infections and as a surface antiseptic .
Nitrofural
| Structural formula with marked remainder and international non-proprietary name | Example of an application area |
|---|---|
 Nitrofural trade name: Furacin-Sol |
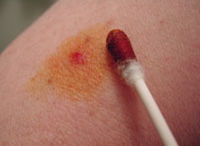 Use as a surface antiseptic |
Nitrofural can be used as a surface antiseptic for the treatment of complicated wound infections and skin diseases .
Nifurtoinol
| Structural formula with marked remainder and international non-proprietary name | Example of an application area |
|---|---|
 Nifurtoinol |
 Combating Giardiasis Pathogens |
Nifurtoinol can be used to combat the flagellate Giardia intestinalis, which is responsible for giardiasis , and to treat urinary tract infections .
Other antibiotic nitrofurans
| Further antibiotic nitrofurans, structural formulas with marked remainder and international non-proprietary names and areas of application | ||
|---|---|---|
|
Nifuroxazide use as bowel - antiseptic . |
Nifurfolin research on drugs to treat hepatitis C . |
Furazolidone treatment of diarrhea , cholera , trichomonads and salmonella poisoning . |
|
Nifurzide control of Escherichia coli . |
Nitrovin Used as an antibiotic growth promoter until 2006 . Since then no longer approved in the European Union . |
Ranbezolid Treatment of staphylococci ( infestation of the skin and mucous membranes). |
Use as an antimicrobial
Some nitrofurans are used in practice as antimicrobials.
Nifuratel
| Structural formula with marked remainder and international non-proprietary name | Example of an application area |
|---|---|
 Nifuratel |
 Combating a Monilinia fungal attack |
Nifuratel acts as a fungicide and microbicide, for example against infestation by trichomonads and monilinia (fruit mummy).
Nifurtimox
| Structural formula with marked remainder and international non-proprietary name | Example of an application area |
|---|---|
 Nifurtimox trade name: Lampit® |
Treatment of Chagas disease (here: right eye) |
Nifurtimox is relevant in the treatment of trypanosome infestation , protozoal infections and Chagas disease .
Other antimicrobiotic nitrofurans
| Further antimicrobiotic nitrofurans, structural formulas with marked remainder and international non-proprietary names and areas of application | ||
|---|---|---|
|
Furaltadon veterinary medicine (combined preparation with spiramycin ) and treatment of urinary tract infections. |
Furazidin treatment of urinary tract infections. |
Furylfuramide Preservative in Japan until the 1970s . |
Individual evidence
- ↑ a b c d e Jürgen Falbe, Manfred Regitz (Ed.): Römpp Chemie Lexikon, 9th edition, Georg Thieme Verlag, Stuttgart 1990, ISBN 3-13-734909-5 , p. 3019.
- ^ Ernst Mutschler, Gerd Geisslinger, Heyo Klaus Kroemer, Sabine Menzel, Peter Ruth: drug effects: textbook of pharmacology, clinical pharmacology and toxicology; with introductory chapters in anatomy, physiology and pathophysiology . 10th edition. WVG, Wissenschaftliche Verlagsgesellschaft, Stuttgart 2013, ISBN 978-3-8047-2898-1 , p. 792 .
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry. Springer-Verlag, Berlin Heidelberg 2013, ISBN 978-3-642-34715-3 , pp. 808-809.
- ↑ a b Axel Kleemann ; Jürgen Engel; Bernhard Kutscher; Dietmar Reichert: Pharmaceutical Substances - Syntheses, Patents and Applications of the most relevant APIs. , 5th edition, Georg Thieme Verlag KG, 2009, ISBN 978-3-13-558405-8 , p. 980.
- ↑ a b c Red List , as of 2013.
- ↑ Axel Kleemann ; Jürgen Engel; Bernhard Kutscher; Dietmar Reichert: Pharmaceutical Substances - Syntheses, Patents and Applications of the most relevant APIs . 5th edition. Georg Thieme Verlag, 2009, ISBN 978-3-13-558405-8 , p. 981.
- ↑ a b J. M. Wright, LA Dunn, P. Upcroft, JA Upcroft: Efficacy of antigiardial drugs. In: Expert Opinion on Drug Safety . Volume 2, Number 6, November 2003, pp. 529-541, PMID 14585063 .
- ↑ a b c Jürgen Falbe, Manfred Regitz (Ed.): Römpp Chemie Lexikon, 9th edition, Georg Thieme Verlag, Stuttgart 1990, ISBN 3-13-734909-5 , p. 3001.
- ↑ Patent US20140030194: Compositions and Methods for the Treatment of Hepatic Diseases and Disorders . Filed January 16, 2013, published January 30, 2013, applicant: Labyrinth Bio, Inc., inventor: Elizabeth McKenna.
- ↑ a b Jürgen Falbe, Manfred Regitz (ed.): Römpp Chemie Lexikon, 9th edition, Georg Thieme Verlag, Stuttgart 1990, ISBN 3-13-734709-2 , p. 1461.
- ↑ Anne Delsarte, Michel Faway, Jean-Marie Frère Jacques Coyette, Claire Michèlle Calber-Bacq, Ernst Heinen: Nifurzide, a nitrofuran anti Infectious Agent: with Interaction Escherichia coli Cells. In: Antimicrobial Agents and Chemotherapy . , Volume 19, No. 3, pp. 477-486, PMID 7018391 , doi: 10.1128 / aac.19.3.477 .
- ^ GN Dutta, LA Devriese, PF van Assche: Susceptibility of clostridia from farm animals to 21 antimicrobial agents including some used for growth promotion. In: The Journal of Antimicrobial Chemotherapy . Vol. 12 (4) 1983, pp. 347-356; PMID 6643330 .
- ↑ Vandana Kalia, Rajni Miglani, Kedar P. Purnapatre, Tarun Mathur, Smita Singhal, Seema Khan, Sreedhara R. Voleti, Dilip J. Upadhyay, Kulvinder Singh Saini, Ashok Rattan, V. Samuel Raj: Mode of Action of Ranbezolid against Staphylococci and Structural Modeling Studies of Its Interaction with Ribosomes. In: Antimicrobial Agents and Chemotherapy . Vol. 53 (4) 2009, pp. 1427-1433; PMID 19075051 .
- ↑ WHO fact sheet on Chagas disease ; Retrieved June 26, 2017.
- ↑ External identifiers of or database links to furaltadon : CAS number: 139-91-3, EC number: 205-384-5, ECHA InfoCard: 100.004.895 , PubChem : 3434 , ChemSpider : 3316 , Wikidata : Q27144914 .
- ↑ P. Männistö, P. Karttunen: Pharmacokinetics of furagin, a new nitrofurantoin congener, on human volunteers. In: International Journal of Clinical Pharmacology and Biopharmacy . 17 (6), 1979, pp. 264-270; PMID 468451 .
- ^ History: 1970s , Consumers Union of Japan; accessed on June 22, 2017.