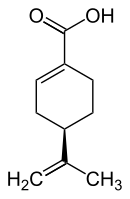
Perillic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| (+) - perilla acid (left) or (-) - perilla acid (right) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Perillic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 14 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 166.21 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Perilla acid is a natural substance . D - (+) - perillic acid has antimicrobial and anti-fungal (" antifungal ") properties and is therefore of great interest to the cosmetics, food and pharmaceutical industries for use as a natural preservative.
Occurrence
L - (-) - perillic acid occurs naturally in the black nettle ( Perilla frutescens ), where it is mainly bound as β-glucoside. It is also found in small amounts in the essential oils of black nettle and lemongrass .
Extraction or representation
The natural occurrences show only a low concentration of perillic acid, so extraction is uneconomical. Alternatively, perillic acid can be synthesized from β- pinene , but this method, too, has a relatively low yield and high cost.
The gram-negative bacterium Pseudomonas putida DSM 12264 can regioselectively oxidize D - (+) - limonene to D - (+) - perillaic acid. The production is demanding from a biotechnological point of view, since the production of perillic acid is inhibited by an excessive concentration of limonene as well as of perillic acid. A bioprocess, which was further developed on a laboratory scale in 2010, represents a promising option for industrial use. The product is continuously removed by an anion exchanger and limonene is added according to consumption.
Isomers
There are two enantiomers , the D - (+) - perillaic acid [also known as ( R ) - (+) - perillaic acid or (+) - perillaic acid for short] and the ( S ) - (-) - perillaic acid [also known as L - (-) - Perillaic acid or (-) - Perillaic acid for short].
Individual evidence
- ↑ Entry on PERILLIC ACID in the CosIng database of the EU Commission, accessed on August 17, 2020.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
-
^ Ruben Eckermann: With bacteria against bacteria . In: News from chemistry . tape 59 , 2011, p. 619-620 .
For the biotechnological production of perillic acid, the working group of industrial research associations "Otto von Guericke" (AiF) awarded the Otto von Guericke Prize 2011 to Jens Schrader from the Karl Winnacker Institute (today DECHEMA Research Institute ).
- ↑ MA Mirata et al .: Integrated bioproduction and selective purification of perillic acid , Chemie Ingenieur Technik , 82 ( 2010 ) pp. 101-109.